Question: I NEED HELP!! From the list below, pick the one species that cannot act as both a Bronsted-Lowry acid and base. A. HCO3 B. H2SO4
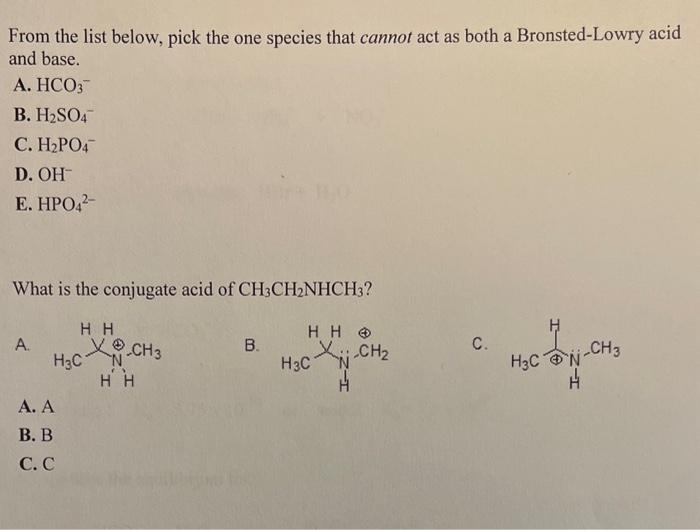
From the list below, pick the one species that cannot act as both a Bronsted-Lowry acid and base. A. HCO3 B. H2SO4 C. H2PO4 D. OH E. HPO42 What is the conjugate acid of CH3CH2NHCH3 ? A. B. C. A. A B. B C. C
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


