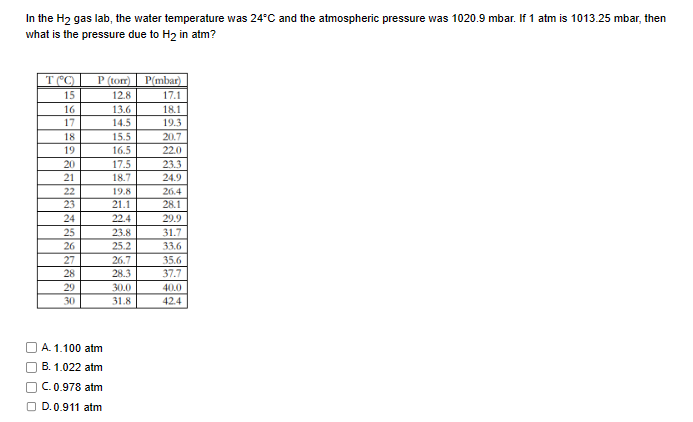
Question: I need help here, BTW 1 . 1 0 0 and 1 . 0 2 2 atm are wrong. In the H 2 gas lab,
I need help here, BTW and atm are wrong.
In the gas lab, the water temperature was and the atmospheric pressure was If atm is then
what is the pressure due to in atm?
Aatm
Batm
Catm
Datm I
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


