Question: I need help I do not understand this A mixture containing KClO3,K2CO3,KHCO3, and How many grams of KClO3 were in the original mixture? KCl was
I need help I do not understand this
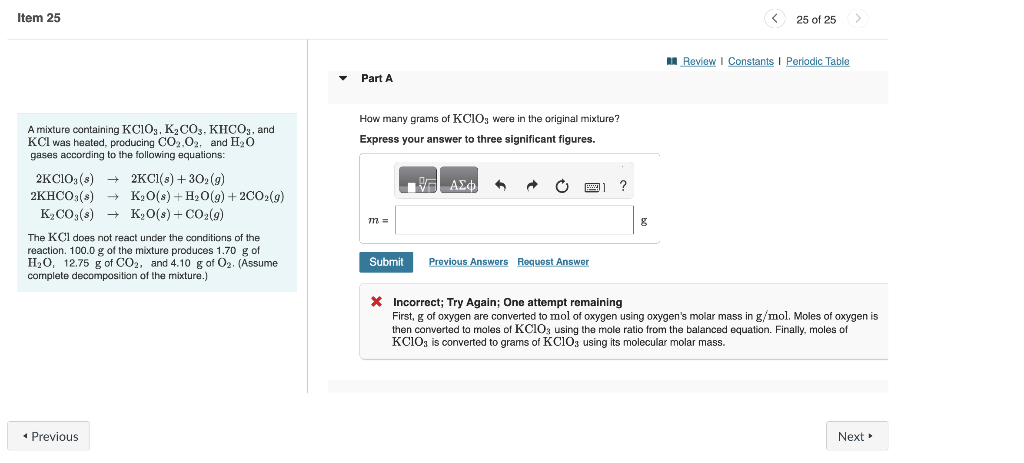
A mixture containing KClO3,K2CO3,KHCO3, and How many grams of KClO3 were in the original mixture? KCl was heated, producing CO2,O2, and H2O Express your answer to three significant figures. gases according to the following equations: 2KClO3(s)2KHCO3(s)K2CO3(s)2KCl(s)+3O2(g)K2O(s)+H2O(g)+2CO2(g)K2O(s)+CO2(g) The KCl does not react under the conditions of the reaction. 100.0g of the mixture produces 1.70g of H2O,12.75g of CO2, and 4.10g of O2. (Assume complete decomposition of the mixture.) * Incorrect; Try Again; One attempt remaining First, g of oxygen are converted to mol of oxygen using oxygen's molar mass in g/mol. Moles of oxygen is then converted to moles of KClO3 using the mole ratio from the balanced equation. Finally, moles of KClO3 is converted to grams of KClO3 using its molecular molar mass
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


