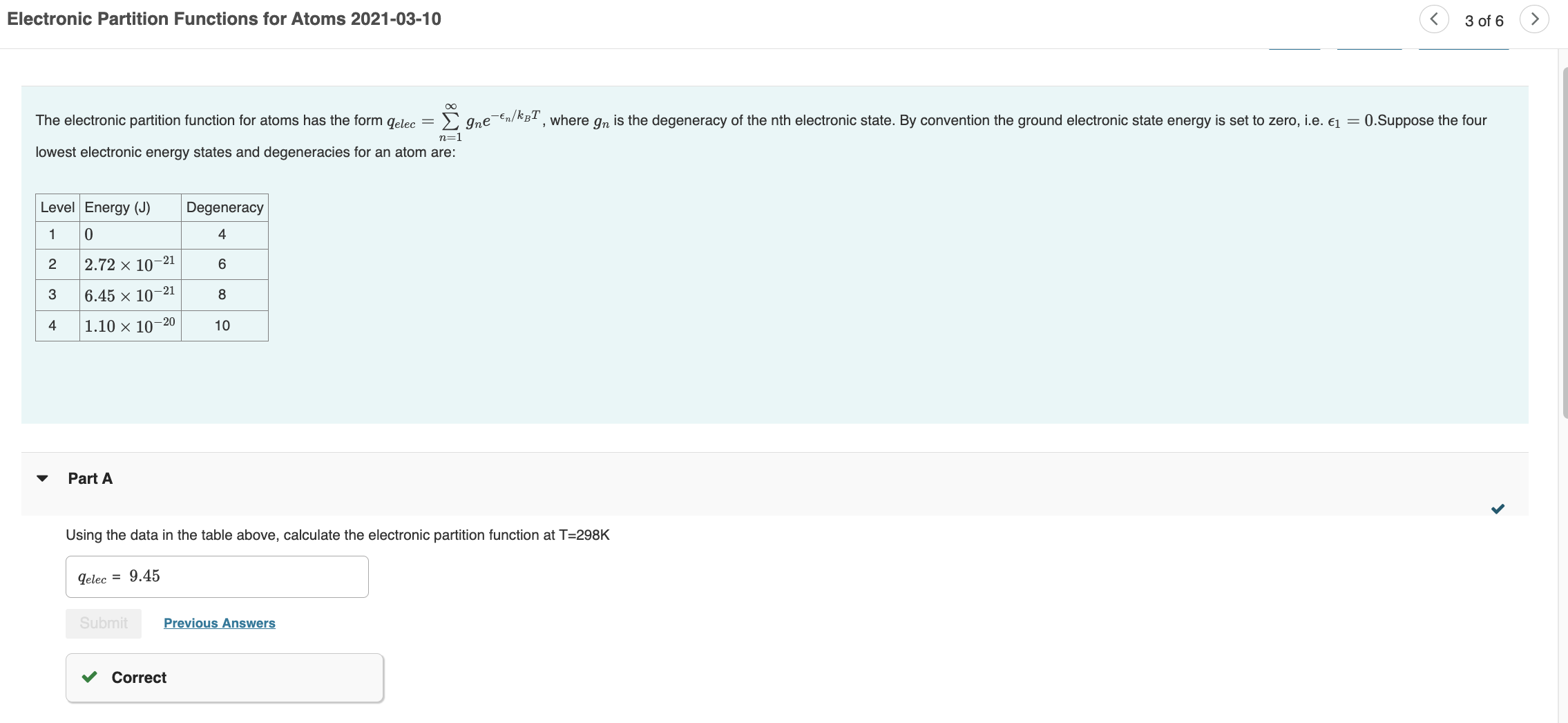
Question: I NEED HELP ON C-E PLEASE! Electronic Partition Functions for Atoms 2021-03-10 3 of 6 lowest electronic energy states and degeneracies for an atom are:
I NEED HELP ON C-E PLEASE!
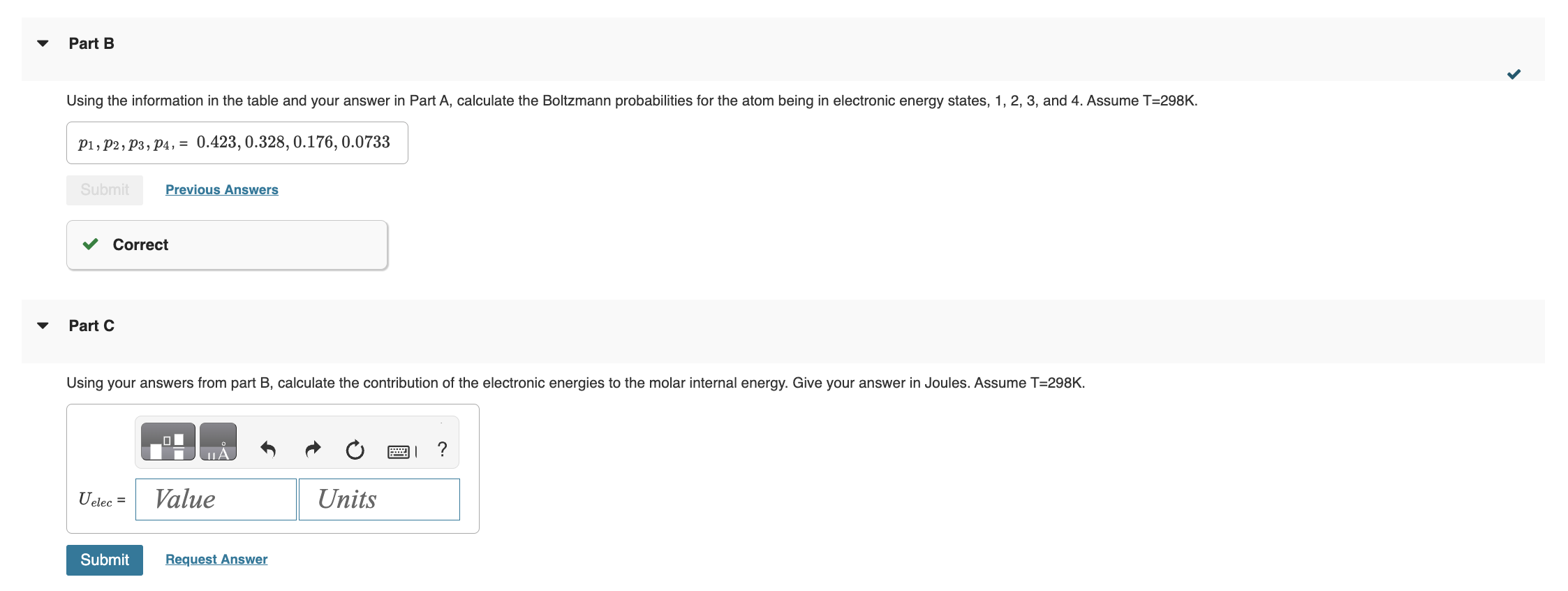
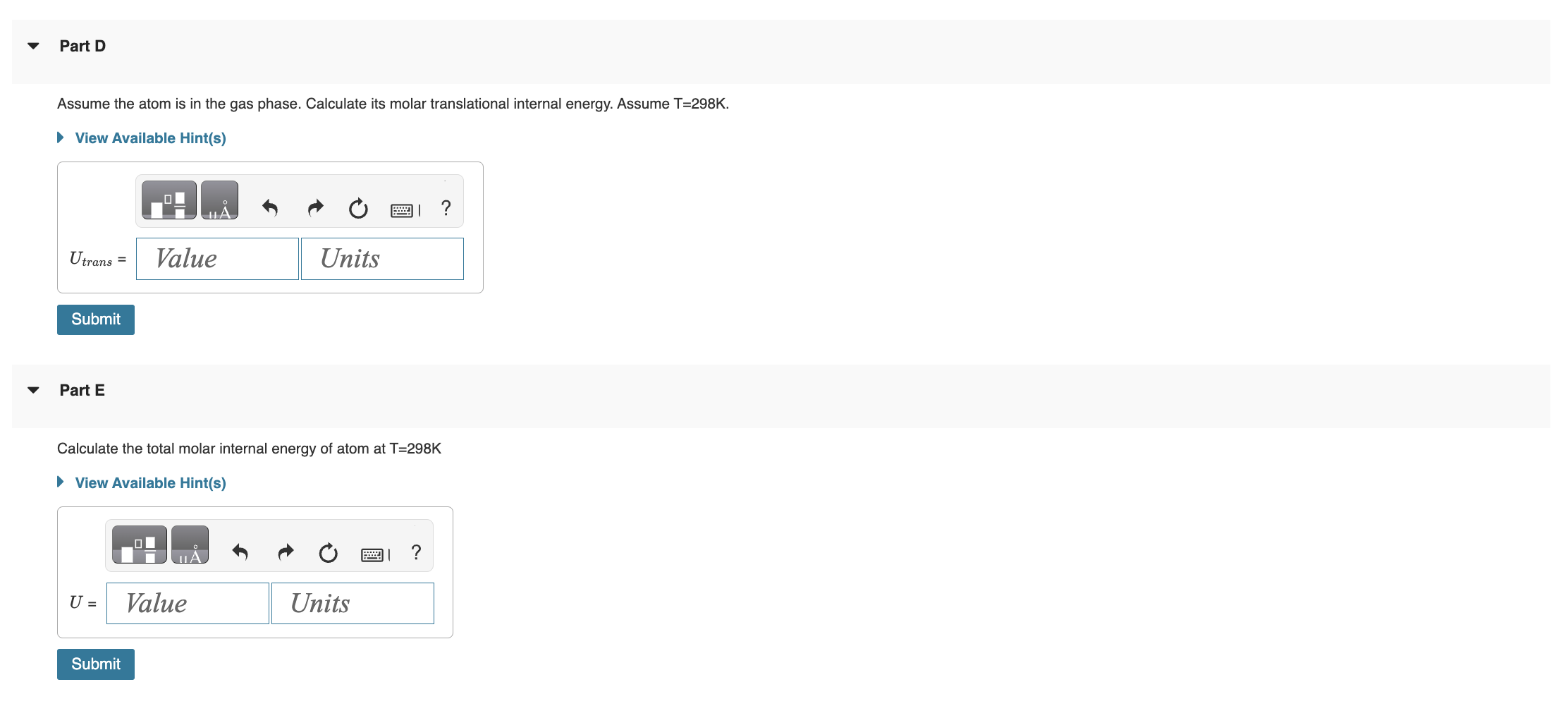
Electronic Partition Functions for Atoms 2021-03-10 3 of 6 lowest electronic energy states and degeneracies for an atom are: Part A Using the data in the table above, calculate the electronic partition function at T=298K Part B Using the information in the table and your answer in Part A, calculate the Boltzmann probabilities for the atom being in electronic energy states, 1, 2, 3, and 4. Assume T=298K. p1,p2,p3,p4,=0.423,0.328,0.176,0.0733 Submit Previous Answers Correct Part C Using your answers from part B, calculate the contribution of the electronic energies to the molar internal energy. Give your answer in Joules. Assume T=298K. Submit Request Answer Assume the atom is in the gas phase. Calculate its molar translational internal energy. Assume T=298K. Part E Calculate the total molar internal energy of atom at T=298K
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


