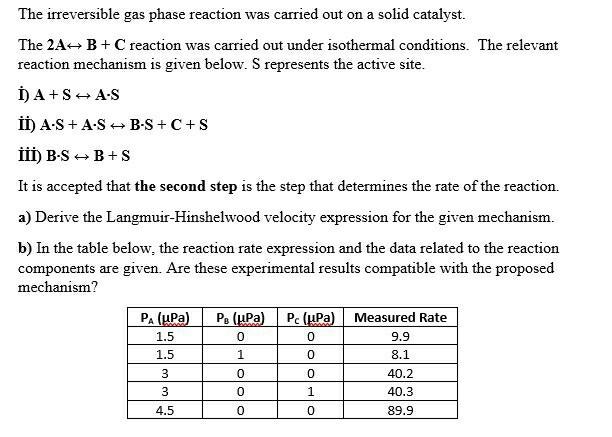
Question: I need help solving this task quickly and thanks The irreversible gas phase reaction was carried out on a solid catalyst. The 2A+ B +
I need help solving this task quickly and thanks
The irreversible gas phase reaction was carried out on a solid catalyst. The 2A+ B + C reaction was carried out under isothermal conditions. The relevant reaction mechanism is given below. S represents the active site. ) A+S + AS ) AS + AS B-S +C+S III) B- SB+S It is accepted that the second step is the step that determines the rate of the reaction. a) Derive the Langmuir-Hinshelwood velocity expression for the given mechanism. b) In the table below, the reaction rate expression and the data related to the reaction components are given. Are these experimental results compatible with the proposed mechanism? P. (Pa) Pc (Pa) PA (upa) 1.5 1.5 0 0 1 Measured Rate 9.9 8.1 40.2 40.3 89.9 0 0 0 1 3 3 4.5 0 0 0
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


