Question: I need help understanding these three question. Please explain why your answer is correct! Complete the following sentence for a ground-state, multi-electron atom. The lower

I need help understanding these three question. Please explain why your answer is correct!
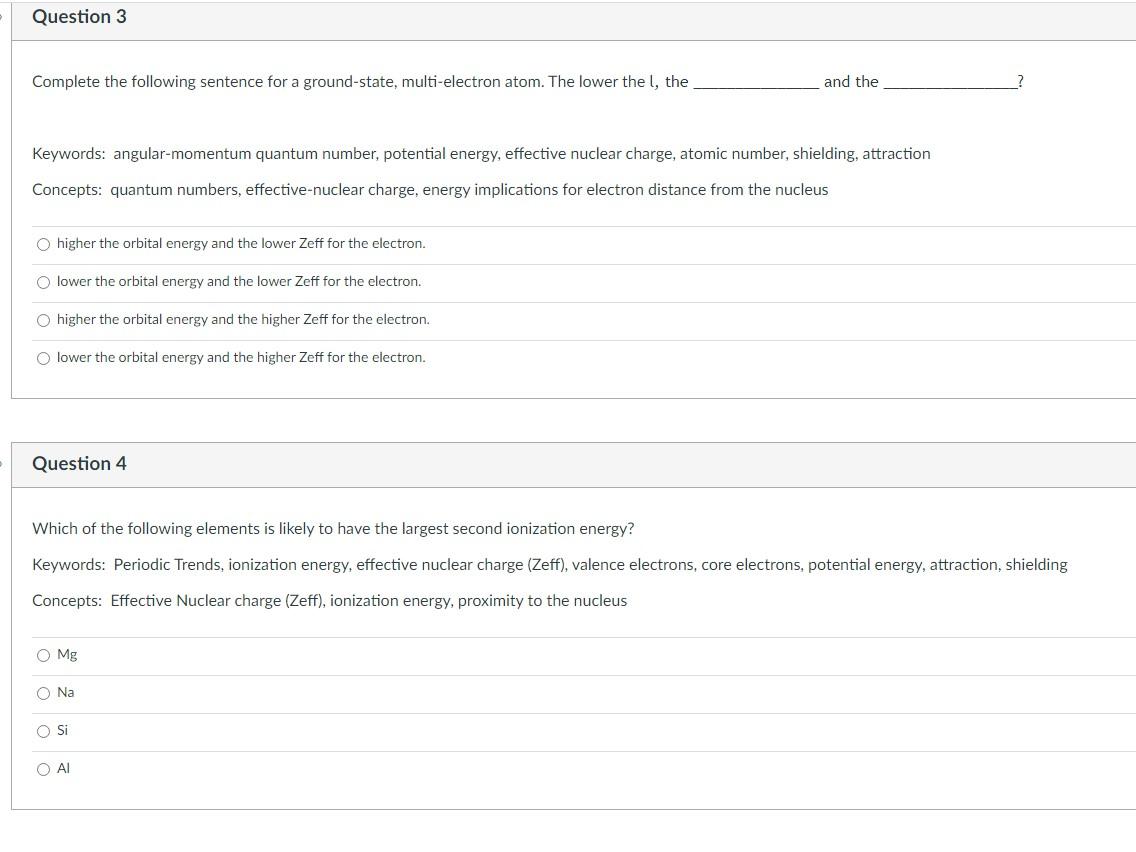
Complete the following sentence for a ground-state, multi-electron atom. The lower the l, the and the Keywords: angular-momentum quantum number, potential energy, effective nuclear charge, atomic number, shielding, attraction Concepts: quantum numbers, effective-nuclear charge, energy implications for electron distance from the nucleus higher the orbital energy and the lower Zeff for the electron. lower the orbital energy and the lower Zeff for the electron. higher the orbital energy and the higher Zeff for the electron. lower the orbital energy and the higher Zeff for the electron. Question 4 Which of the following elements is likely to have the largest second ionization energy? Keywords: Periodic Trends, ionization energy, effective nuclear charge (Zeff), valence electrons, core electrons, potential energy, attraction, shielding Concepts: Effective Nuclear charge (Zeff), ionization energy, proximity to the nucleus Mg Na Si Al How does electron affinity correlate with atomic radius? Keywords: electron affinity, nonmetal, metal, atomic radius, effective nuclear charge (Zeff), principal quantum number, electron, anion Concepts: Electron affinity, atomic radius trend, metallic character B. electron affinity becomes more negative as radii decrease D. electron affinity becomes more positive as radii increase A. electron affinity becomes more negative as radii increase C. electron affinity becomes more positive as radii decrease
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


