Question: i need help with #4 and 5 please, show work Thermochemical data ( at T=25.0C ) is given below, and may be used to do
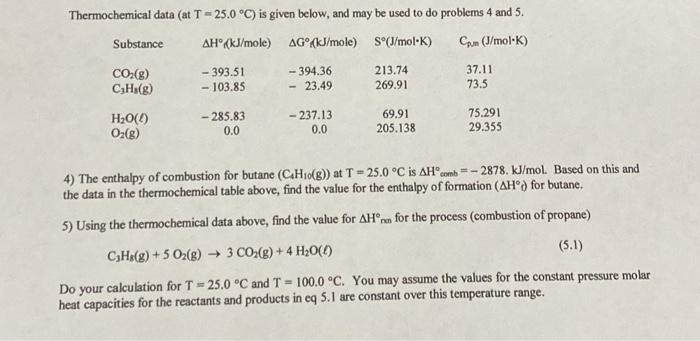
Thermochemical data ( at T=25.0C ) is given below, and may be used to do problems 4 and 5 . 4) The enthalpy of combustion for butane (C4H10(g)) at T=25.0C is Hcomb=2878.kJ/mol. Based on this and the data in the thermochemical table above, find the value for the enthalpy of formation (Hd) for butane. 5) Using the thermochemical data above, find the value for Hnn for the process (combustion of propane) C3H8(g)+5O2(g)3CO2(g)+4H2O() Do your calculation for T=25.0C and T=100.0C. You may assume the values for the constant pressure molar heat capacities for the reactants and products in eq 5.1 are constant over this temperature range
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


