Question: I NEED HELP WITH BOTH ! PLEASE! Suppose you burn 0.212g of C(s) in an excess of O2(g) in a constant V calorimeter to give
I NEED HELP WITH BOTH ! PLEASE!

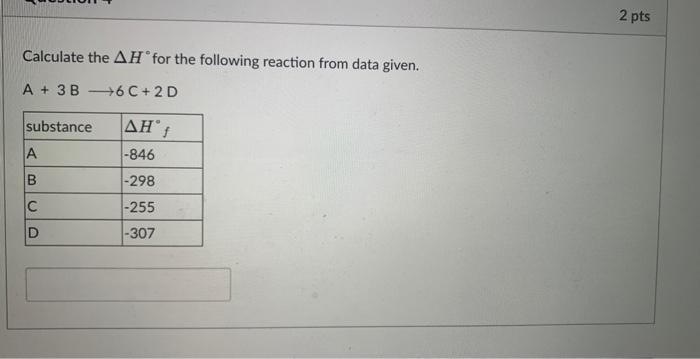
Suppose you burn 0.212g of C(s) in an excess of O2(g) in a constant V calorimeter to give CO2(g). C(s)+O2(g)CO2(g) The T of the calorimeter, which contained 966g of water, increased from 20.00 to 26.71C. The heat capacity of the bomb is 890J/K. Calculate E per mole of carbon (enter in kJ/mol).4.184J/gC for H2O. Enter with correct sign and to 1 decimal place Calculate the H for the following reaction from data given. A+3B6C+2D
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


