Question: I need help with C and D. I would really appreciate it if you can hand write it because I don't understand when you type
I need help with C and D. I would really appreciate it if you can hand write it because I don't understand when you type it out. I like to see the steps and understand. Thank you!
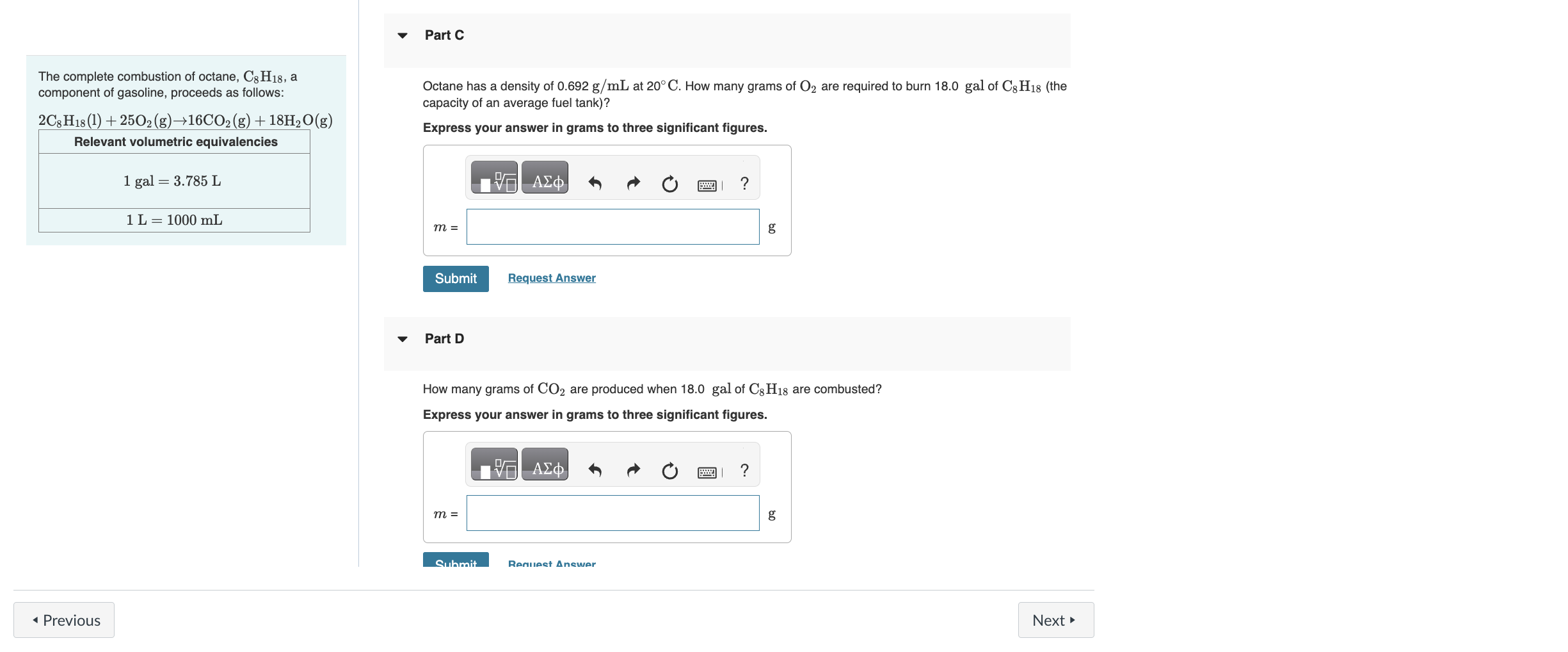
The complete combustion of octane, C8H18, a component of gasoline, proceeds as follows: Octane has a density of 0.692g/mL at 20C. How many grams of O2 are required to burn 18.0 gal of C8H18 (the capacity of an average fuel tank)? 2C8H18(l)+25O2(g)16CO2(g)+18H2O(g) Express your answer in grams to three significant figures. Relevant volumetric equivalencies 1gal=3.785L1L=1000mL Part D How many grams of CO2 are produced when 18.0 gal of C8H18 are combusted? Express your answer in grams to three significant figures
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


