Question: I need help ASAP!!! I would really appreciate it if you can hand write it because I don't understand when you type it out. I
I need help ASAP!!! I would really appreciate it if you can hand write it because I don't understand when you type it out. I like to see the steps and understand. Thank you!
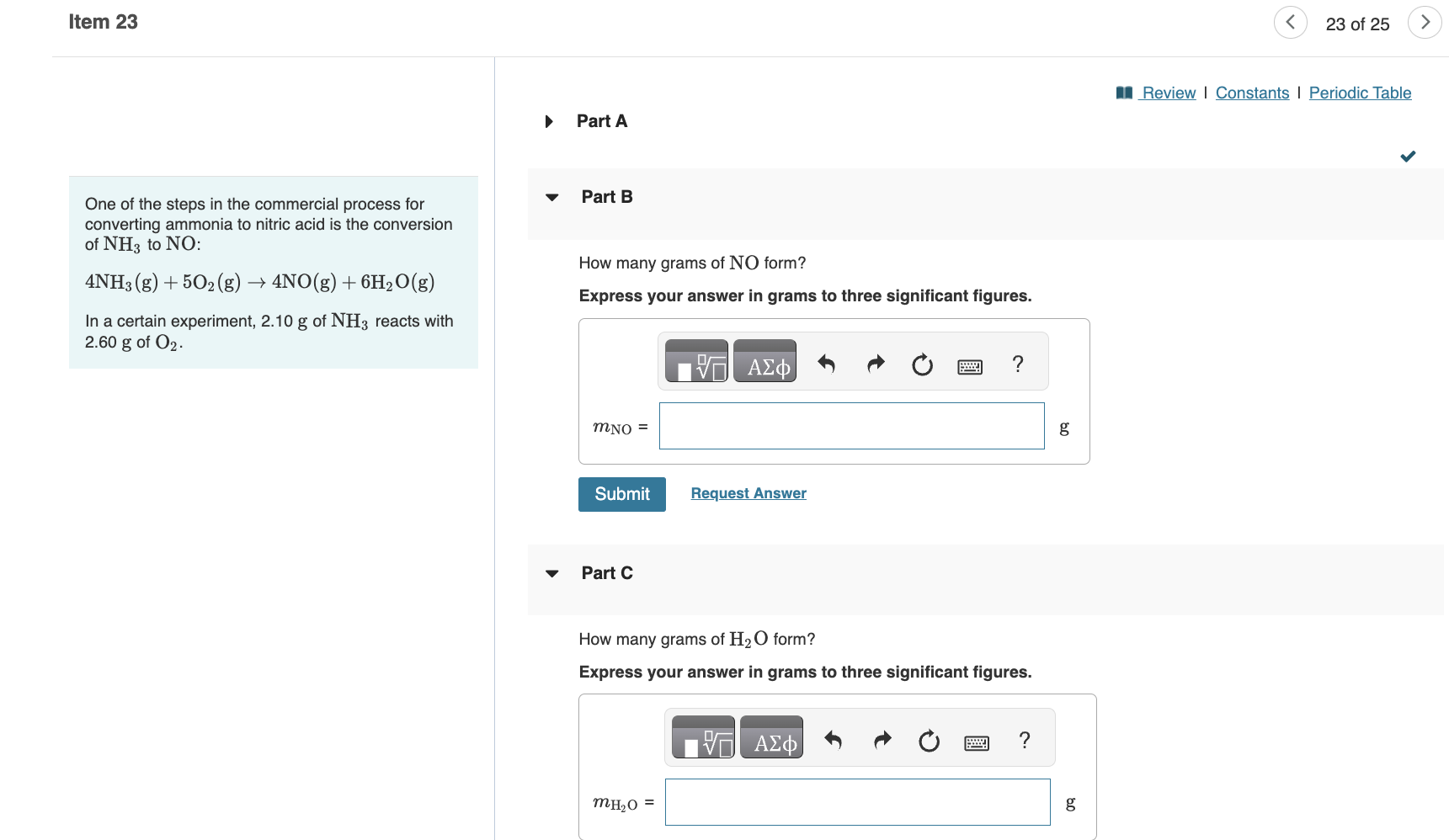
One of the steps in the commercial process for Part B converting ammonia to nitric acid is the conversion of NH3 to NO : 4NH3(g)+5O2(g)4NO(g)+6H2O(g) How many grams of NO form? Express your answer in grams to three significant figures. In a certain experiment, 2.10g of NH3 reacts with 2.60g of O2. Part C How many grams of H2O form? Express your answer in grams to three significant figures
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


