Question: I need help with part C and D using the answers from part A and B. Information : Fritz Haber was awarded a Nobel Prize
I need help with part C and D using the answers from part A and B.
Information: Fritz Haber was awarded a Nobel Prize for the processes he invented in which nitrogen and hydrogen gases are combined to make ammonia (NH3NH3), a valuable chemical and a vital nutrient in modern agriculture. It is known that ammonia is 82.2%% nitrogen by mass.
Part A: If 27.3g of nitrogen with 5.91g of Hydrogen are combined in a closed system, what mass of Ammonia will be produced?
Answer: 33.2g NH3
Part B: What mass of Nitrogen is required to fully consume 11.5 g of hydrogen?
Answer: 53.2g N
Part C:
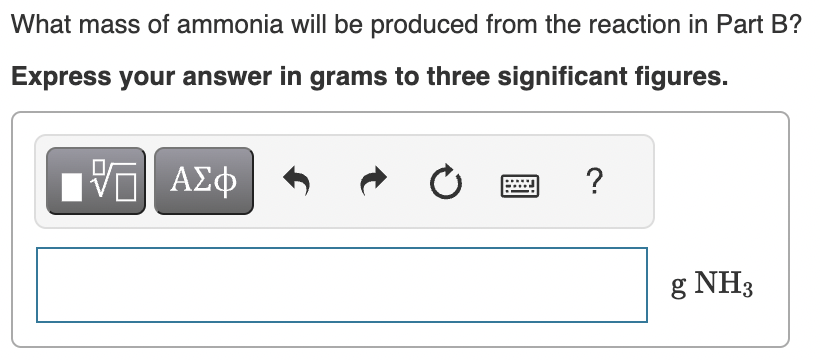
Part D:
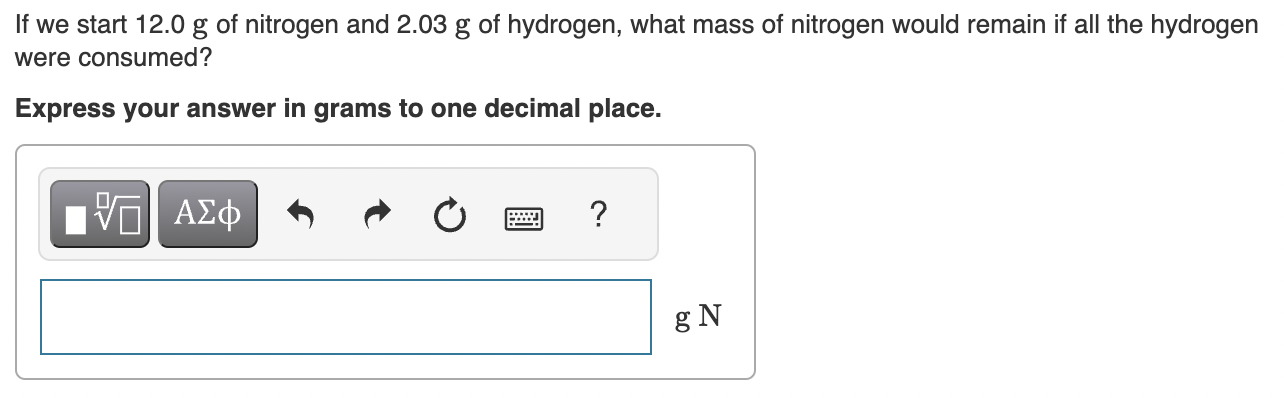
What mass of ammonia will be produced from the reaction in Part B? Express your answer in grams to three significant figures. If we start 12.0g of nitrogen and 2.03g of hydrogen, what mass of nitrogen would remain if all the hydrogen were consumed? Express your answer in grams to one decimal place
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


