Question: I need help with question 2-6. the table is provided with the trials Volume Used (mL) Volume of Mystery Acid (mL) 100 Concentration of KOH
I need help with question 2-6. the table is provided with the trials
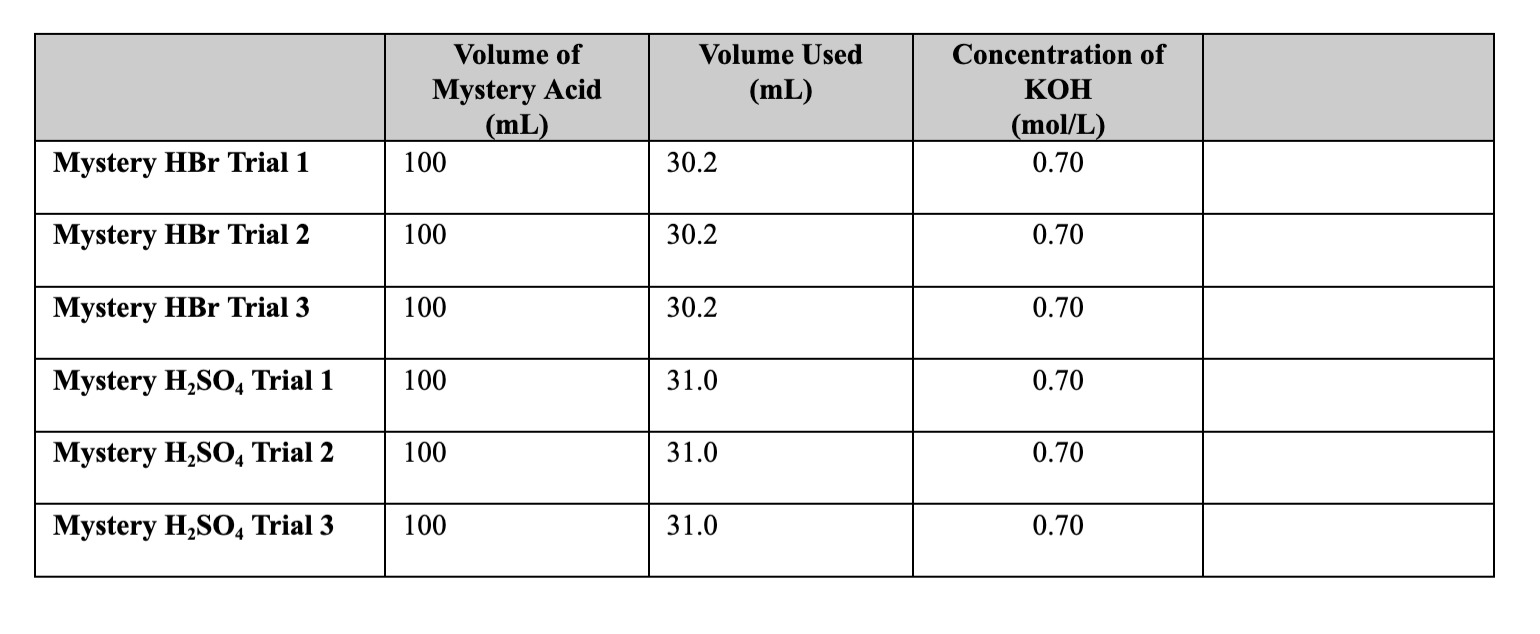


Volume Used (mL) Volume of Mystery Acid (mL) 100 Concentration of KOH (mol/L) 0.70 Mystery HBr Trial 1 30.2 Mystery HBr Trial 2 100 30.2 0.70 Mystery HBr Trial 3 100 30.2 0.70 Mystery H2SO4 Trial 1 100 31.0 0.70 Mystery H2SO4 Trial 2 100 31.0 0.70 Mystery H2SO4 Trial 3 100 31.0 0.70 Calculations - Show all work and units. 1. Write the balanced equation for the reactions that occurred. HBr + KOH = KBr + H2O H2SO4 + 2KOH = K2SO4 + 2H2O 2. Determine the molarity of mystery HBr solution. Use the average value between all your trials. 3. Determine the molarity of Mystery H2SO4 solution. Use the average value between all your trials. 4. Why is an acid/base indicator used during titration? Why is phenolphthalein a good choice for this lab? 5. What are 2 sources of error in this lab? Do not indicate anything that is human error. 6. If 30.0 ml of 0.500 M Ba(OH)2 is needed to neutralize 10.0 ml of HCl of unknown concentration, how many grams of HC1 are in the HCl solution
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


