Question: i need help with the five question does anyone know this please help and also write nadlie please write neatly please Calculations: 1. For each

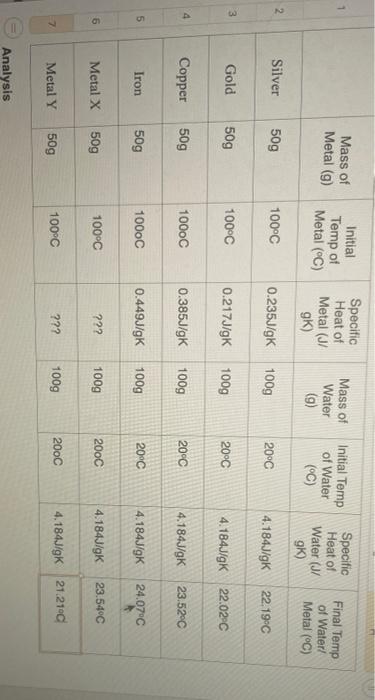

Calculations: 1. For each metal, calculate the heat gained (in joules) by the water. Show all Work! 2. For each metal calculate the heat lost in Joules) by the metal. Show all Work! I Mass of Metal (9) Initial Temp of Metal (C) Specific Heat of Metal (J/ GK) Mass of Water (9) Initial Temp of Water Specific Heat of Water (J/ g) Final Temp of Water Metal (C) (C) 2 Silver 50g 100C 0.235J/gK 100g 2000 4.184J/gK 22.19C 3 Gold 509 100C 0.217J/gK 100g 20C 4.184J/gK 22.02C 4 Copper 509 1000C 0.385J/gK 100g 20C 4.184J/gK 23.52C 5 Iron 50g 1000C 0.449J/gK 100g 20C 4.184J/gK 24.07C 6 Metal x 50g 100C 222 100g 200C 4.184J/gK 23.54C 7 Metal Y 200C 100C 50g ??? 100g 4.184J/gK 21.2100 Analysis 1. Compare the answers for the heat lost and heat gained for each metal/water combination Do they agree? Should they? Justify your answer. If they do not agree, list potential sources of error for this experiment in the real laboratory 2. Calculate the specific heat for the unknowns. I Using the table of specific heat values provided, determine the identity of the unknowns. 3. Discuss the relationship between the specific heat of the various metals and the amount of temperature change they had on the water. Your answer should include a general correlation and an explanation of why
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


