Question: i need help with the second part, the first picture is so that it can help you with the equation. Determine the balanced chemical equation
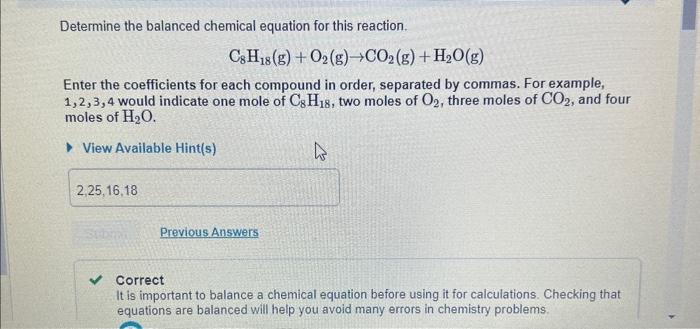
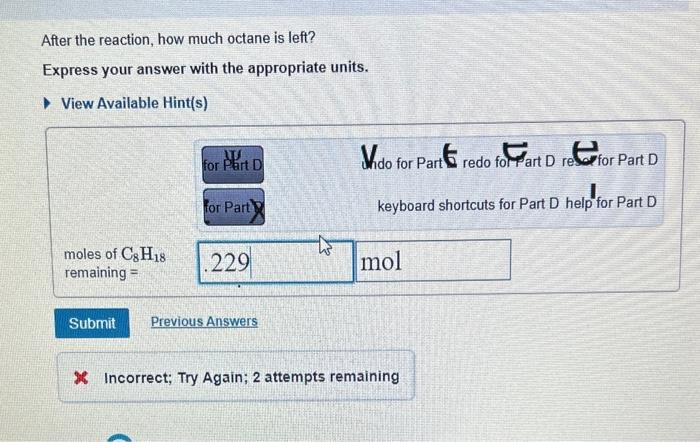
Determine the balanced chemical equation for this reaction. C8H18(g)+O2(g)CO2(g)+H2O(g) Enter the coefficients for each compound in order, separated by commas. For example, 1,2,3,4 would indicate one mole of C8H18, two moles of O2, three moles of CO2, and four moles of H2O. View Available Hint(s) Correct It is important to balance a chemical equation before using it for calculations. Checking that equations are balanced will help you avoid many errors in chemistry problems. After the reaction, how much octane is left? Express your answer with the appropriate units. View Available Hint(s) Wido for Part 6 redo for art D rest fart D keyboard shortcuts for Part D help for Part D
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


