Question: I need help with the solution flash point. The flash point of a liquid is the minimum temperature at which the vapor above that liquid
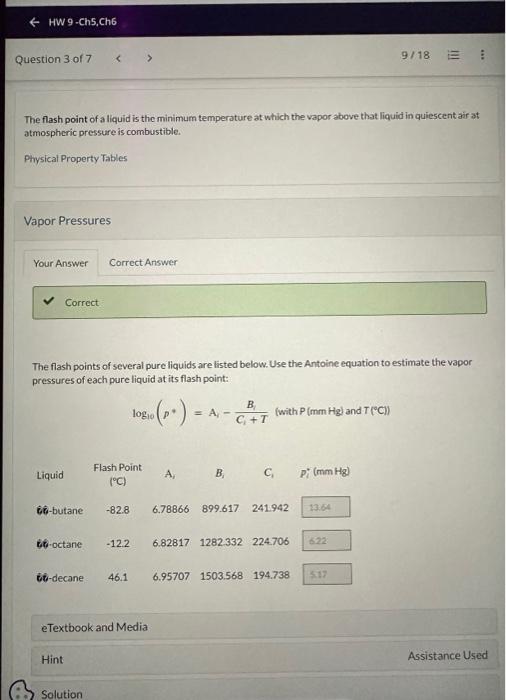
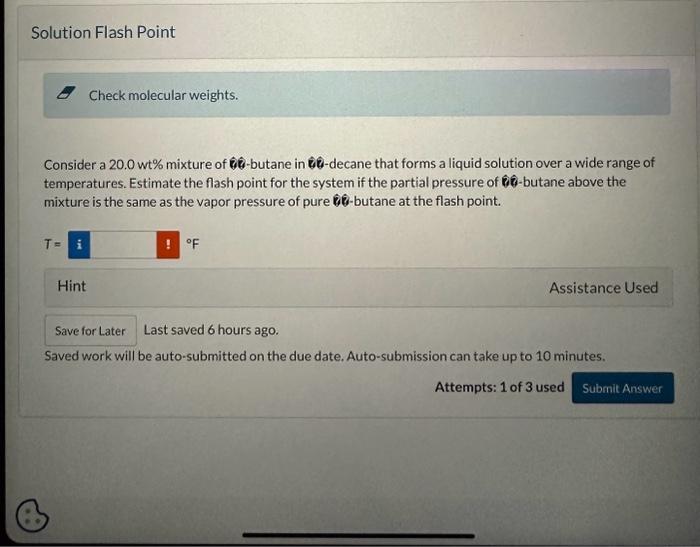
The flash point of a liquid is the minimum temperature at which the vapor above that liquid in quiescent air at atmospheric pressure is combustible. Physical Property Tables Vapor Pressures The flash points of several pure liquids are listed below. Use the Antoine equation to estimate the vapor pressures of each pure liquid at its flash point: log10(p)=AiCi+TBi(withP(mmHgi)andT(C)) E Check molecular weights. Consider a 20.0 wt\% mixture of 0-butane in 80-decane that forms a liquid solution over a wide range of temperatures. Estimate the flash point for the system if the partial pressure of 0-butane above the mixture is the same as the vapor pressure of pure 60 -butane at the flash point. T=F Last saved 6 hours ago. Saved work will be auto-submitted on the due date. Auto-submission can take up to 10 minutes
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


