Question: I need help with these questions asap please. Problem 1 (a) Determine the number of valence electrons present in the molecular formula below. CHIN No.
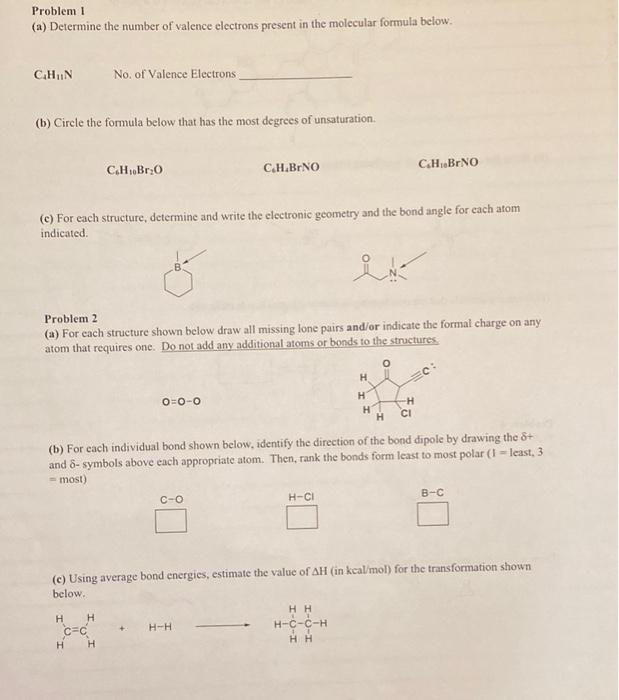
Problem 1 (a) Determine the number of valence electrons present in the molecular formula below. CHIN No. of Valence Electrons (b) Circle the formula below that has the most degrees of unsaturation: C.H.Br 0 C.H.BINO C.H.BrNO (c) For each structure, determine and write the electronic geometry and the bond angle for cach atom indicated Problem 2 (a) For each structure shown below draw all missing lone pairs and/or indicate the formal charge on any atom that requires one. Do not add any additional atoms or bonds to the structures 0 H O=O-O H H H -H CI (b) For each individual bond shown below, identify the direction of the bond dipole by drawing the 8+ and 6- symbols above each appropriate atom. Then, rank the bonds form least to most polar (1 - least, 3 - most) H-CI B-C C-O (c) Using average bond energies, estimate the value of AH (in kcal/mol) for the transformation shown below. + H-H H H CEC H H HH H-C-C-H HH
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


