Question: pls answer this for me this is for a lab. Please help me answer them I need to answer the Energy level Diagram 1 ENERGY
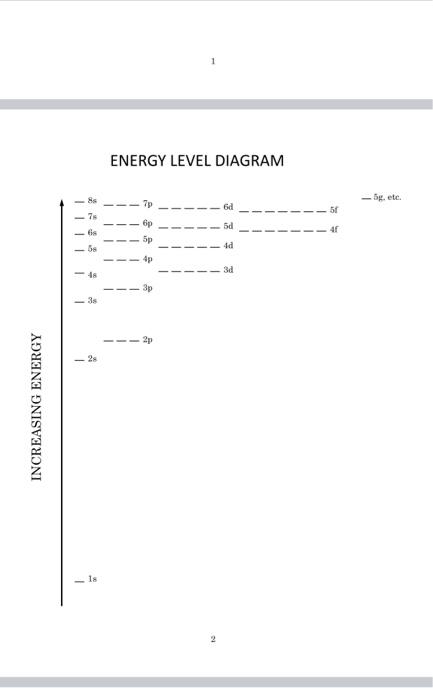

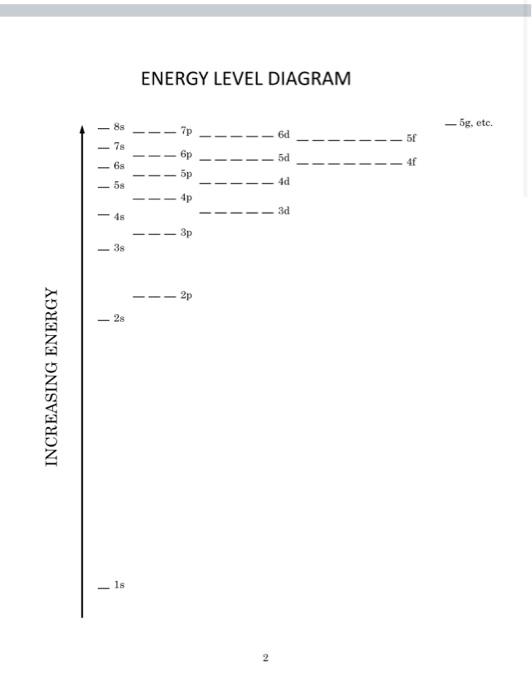
1 ENERGY LEVEL DIAGRAM - gete. d 4 tis) 58 5p 4d 4p 3d 48 3p -3 2p INCREASING ENERGY 18 ELECTRONS AND CHEMISTRY INTRODUCTION This exercise is intended to provide a sample of how the quantum mechanics model of electron configurations can be related to the chemical properties of the elements. From descriptive chemistry, we already know the common charges of many elements that form ions and the ratios with which elements combine in simple compounds. You will be asked to use an electron energy level diagram to examine electron configurations and account for these properties ELECTRONIC CONFIGURATIONS On of the most important conclusions of quantum theory is that electrons in atoms must occupy one of the distinct energy states allowed by certain quantization rules. The rules determine what states are possible. When we assign each state an energy and rank the energies, we create the energy level diagram. This diagram can be used as a tool to make a model of how all the electrons in a neutral atom might be arranged so that no two share the same state and the overall arrangement has the lowest possible potential energy. This arrangement of electrons is called the ground-state configuration. We construct the configuration by placing electrons - symbolized by arrows to indicate up" or "down" spin into the horizontal spaces that represent orbitals. Each orbital can contain at most two electrons (if they have opposite spin). Why is this model of the electron arrangement important? It is because this model allows us to focus on what electrons are at the greatest average distance from the nucleus. These are the electrons that share the highest principle quantum number. They are called the valence electrons. The valence, or outermost, electrons would be the first to come into contact with other atoms during reactions. The number of valence electrons present or the number of vacancies where more could be added are important in predicting the properties of the elements. It is the ability to predict the number of valence electrons in atoms of the different elements that allowed scientists to explain the chemical properties of the elements and the periodic law. 1 ENERGY LEVEL DIAGRAM - 5g, etc. 7s 6p 6s -58 4d 4p 3d - 4s 38 2p 2s INCREASING ENERGY Is 2 1 ENERGY LEVEL DIAGRAM - gete. d 4 tis) 58 5p 4d 4p 3d 48 3p -3 2p INCREASING ENERGY 18 ELECTRONS AND CHEMISTRY INTRODUCTION This exercise is intended to provide a sample of how the quantum mechanics model of electron configurations can be related to the chemical properties of the elements. From descriptive chemistry, we already know the common charges of many elements that form ions and the ratios with which elements combine in simple compounds. You will be asked to use an electron energy level diagram to examine electron configurations and account for these properties ELECTRONIC CONFIGURATIONS On of the most important conclusions of quantum theory is that electrons in atoms must occupy one of the distinct energy states allowed by certain quantization rules. The rules determine what states are possible. When we assign each state an energy and rank the energies, we create the energy level diagram. This diagram can be used as a tool to make a model of how all the electrons in a neutral atom might be arranged so that no two share the same state and the overall arrangement has the lowest possible potential energy. This arrangement of electrons is called the ground-state configuration. We construct the configuration by placing electrons - symbolized by arrows to indicate up" or "down" spin into the horizontal spaces that represent orbitals. Each orbital can contain at most two electrons (if they have opposite spin). Why is this model of the electron arrangement important? It is because this model allows us to focus on what electrons are at the greatest average distance from the nucleus. These are the electrons that share the highest principle quantum number. They are called the valence electrons. The valence, or outermost, electrons would be the first to come into contact with other atoms during reactions. The number of valence electrons present or the number of vacancies where more could be added are important in predicting the properties of the elements. It is the ability to predict the number of valence electrons in atoms of the different elements that allowed scientists to explain the chemical properties of the elements and the periodic law. 1 ENERGY LEVEL DIAGRAM - 5g, etc. 7s 6p 6s -58 4d 4p 3d - 4s 38 2p 2s INCREASING ENERGY Is 2
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


