Question: I need help with these questions please! 2. the concentrations of the products in the reaction below increase or decrease if the initial concentrations are
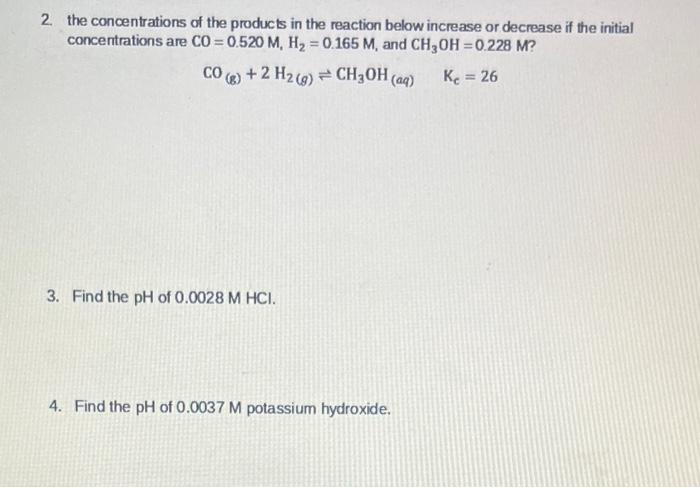
2. the concentrations of the products in the reaction below increase or decrease if the initial concentrations are CO=0.520M,H2=0.165M, and CH3OH=0.228M ? CO(g)+2H2(g)CH3OH(aq)Kc=26 3. Find the pH of 0.0028MHCl. 4. Find the pH of 0.0037M potassium hydroxide
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


