Question: I need help with this multiple part question. The previous model it refers to is ct+1 = (1 ? q)ct + q?. (3) Our previous
I need help with this multiple part question. The previous model it refers to is ct+1 = (1 ? q)ct + q?.
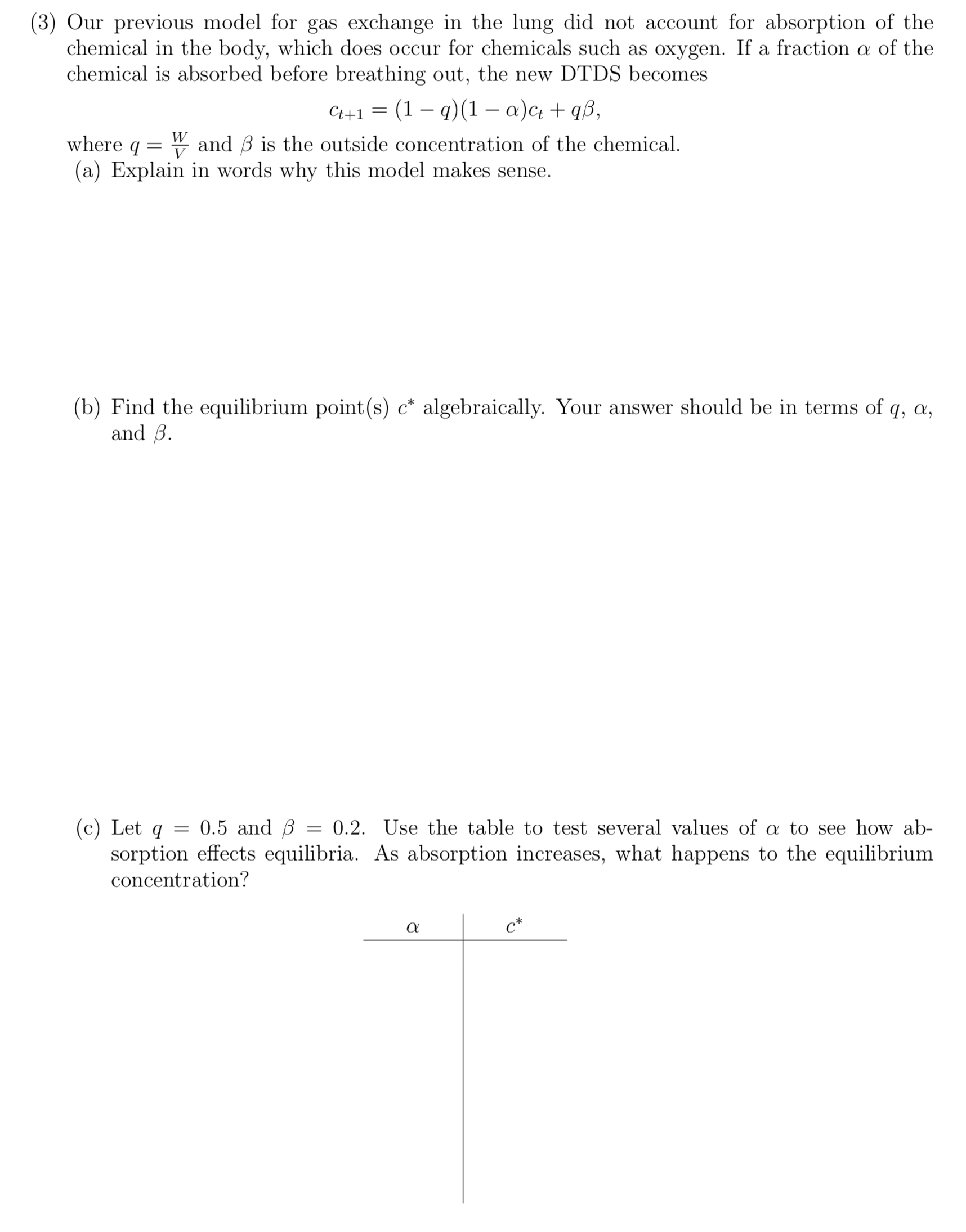
(3) Our previous model for gas exchange in the lung did not account for absorption of the chemical in the body, which does occur for chemicals such as oxygen. If a fraction (1 of the chemical is absorbed before breathing out, the new DTDS becomes Ct+1 = (1 - t1)(1 \"M + '15, where q = g and is the outside concentration of the chemical. (a) Explain in words why this model makes sense. (b) Find the equilibrium point(s) 0* algebraically. Your answer should be in terms of q, a, and . ((2) Let q = 0.5 and ,6 = 0.2. Use the table to test several values of a to see how ab- sorption effects equilibria. As absorption increases, what happens to the equilibrium concentration
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


