Question: I need help with this question 1. Consider the following changes a H2O ------> H2Om b. Haig) + Cl2(g) ----> 2HCl) c. 2H2(g) + 026)
I need help with this question 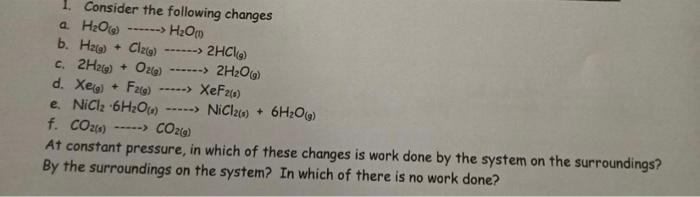
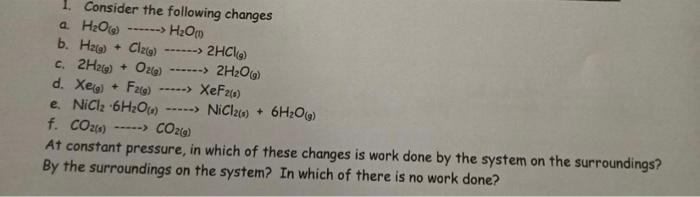
1. Consider the following changes a H2O ------> H2Om b. Haig) + Cl2(g) ----> 2HCl) c. 2H2(g) + 026) ------> 2H2O(39) d. Xeo) + F2(g) -----> XeF26) e. NiClz 6H2O() --..-> NiCl20) + 6H2O(9) f. CO26) -----> CO2(0) At constant pressure, in which of these changes is work done by the system on the surroundings? By the surroundings on the system? In which of there is no work done
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


