Question: I need help with this question. ?How much heat is required to convert 28.1g of liquid H2O at 71C into steam at 133.8C For liquid
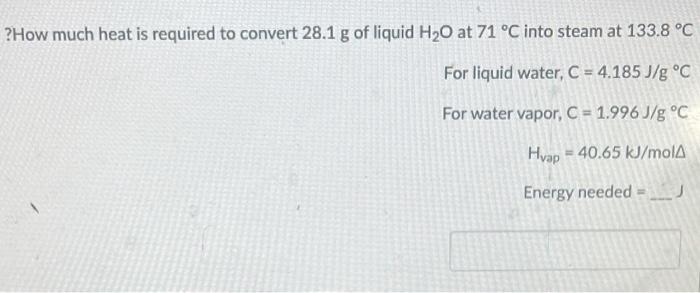
?How much heat is required to convert 28.1g of liquid H2O at 71C into steam at 133.8C For liquid water, C=4.185J/gC For water vapor, C=1.996J/gC Hvap=40.65kJ/mol Energy needed =
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


