Question: How much heat is required to convert 2 0 . 4 g of liquid H 2 O at 8 3 . 8 deg C
How much heat is required to convert g of liquid HO at deg C into steam at deg C
For liquid water, C Jg deg C
For water vapor, C Jg deg C
Hvap kJmol
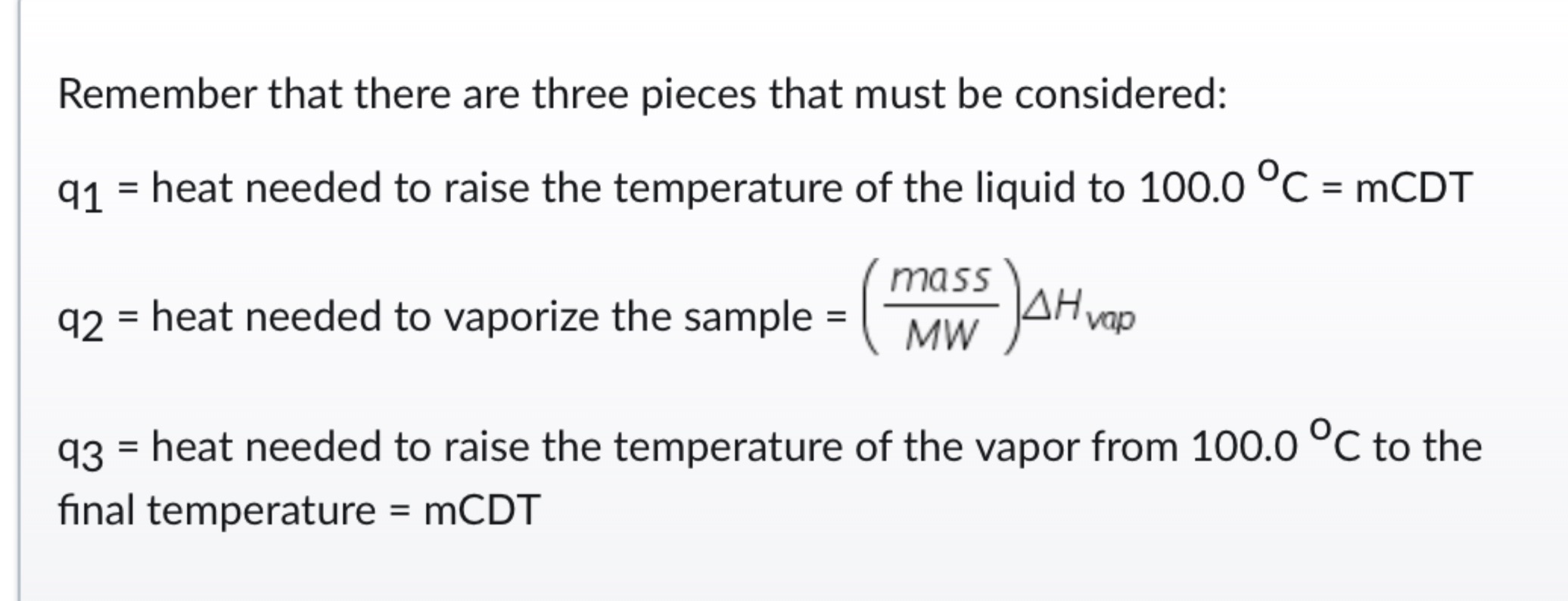
Energy needed JRemember that there are three pieces that must be considered:
heat needed to raise the temperature of the liquid to
heat needed to vaporize the sample
heat needed to raise the temperature of the vapor from to the
final temperature
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


