Question: i need it right now Given that the formula weight (FW) of glucose (C6H12O) is 180 amu, what is the mass in (in grams) of
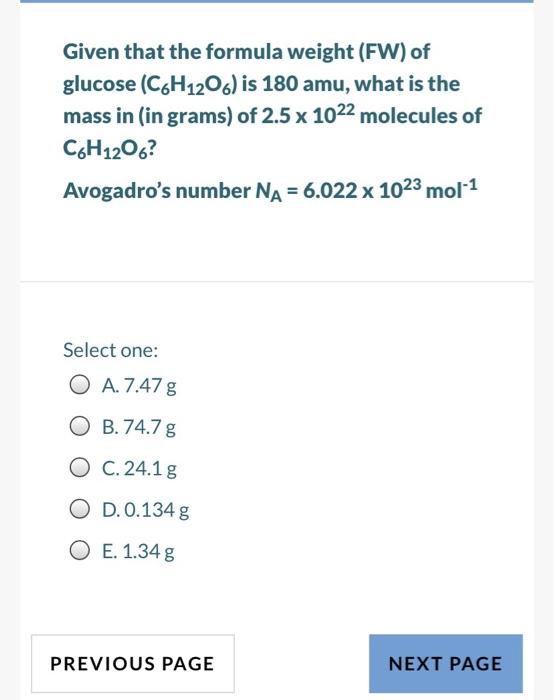
Given that the formula weight (FW) of glucose (C6H12O) is 180 amu, what is the mass in (in grams) of 2.5 x 1022 molecules of C6H1206? Avogadro's number NA = 6.022 x 1023 mol-1 Select one: O A.7.47 g O B. 74.7 g O C. 24.1 g O D. 0.134 g O E. 1.34 g PREVIOUS PAGE NEXT PAGE
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


