Question: 3.(6) Calculate the activation energy, Ea, in kJ/mol for the following reaction from the observed rate constants K25c = 3.46 X 105 s and
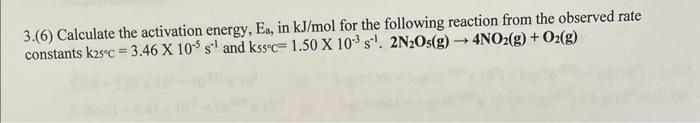
3.(6) Calculate the activation energy, Ea, in kJ/mol for the following reaction from the observed rate constants K25c = 3.46 X 105 s and kssc= 1.50 X 10 s. 2N2Os(g) 4NO2(g) + O2(g) 3.(6) Calculate the activation energy, Ea, in kJ/mol for the following reaction from the observed rate constants k25c = 3.46 X 105 s and kssc= 1.50 X 10 s. 2N2Os(g) 4NO2(g) + O2(g)
Step by Step Solution
3.28 Rating (157 Votes )
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


