Question: I need the answer as soon as possible Q.1. The vapor pressure of 1-chlorotetradecane at several temperature are tabulated here: T(C) P (mm Hg) 98.5
I need the answer as soon as possible 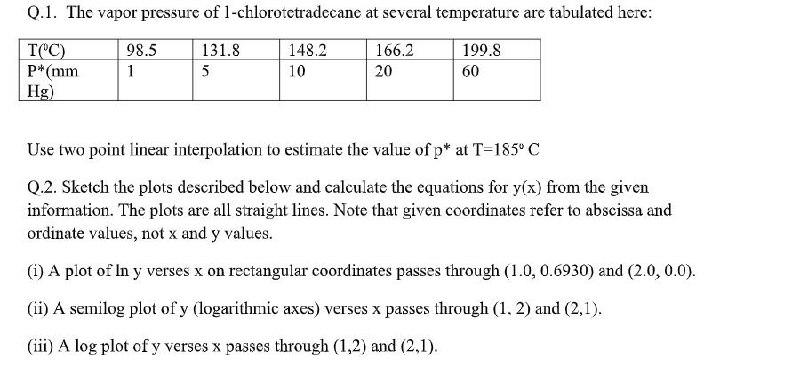
Q.1. The vapor pressure of 1-chlorotetradecane at several temperature are tabulated here: T("C) P" (mm Hg) 98.5 1 131.8 5 148.2 10 166.2 20 199.8 60 Use two point linear interpolation to estimate the value of p* at T=185C Q.2. Sketch the plots described below and calculate the equations for y(x) from the given information. The plots are all straight lines. Note that given coordinates refer to abscissa and ordinate values, not x and y values. (1) A plot of In y verses x on rectangular coordinates passes through (1.0, 0.6930) and (2.0, 0.0). (ii) A semilog plot of y (logarithmic axes) verses x passes through (1, 2) and (2,1). (iii) A log plot of y verses x passes through (1,2) and (2,1)
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


