Question: I need the answer as soon as possible Q.5. Methanol can be produced by the reaction of carbon dioxide and hydrogen CO2 + 3H, CH-OH
I need the answer as soon as possible 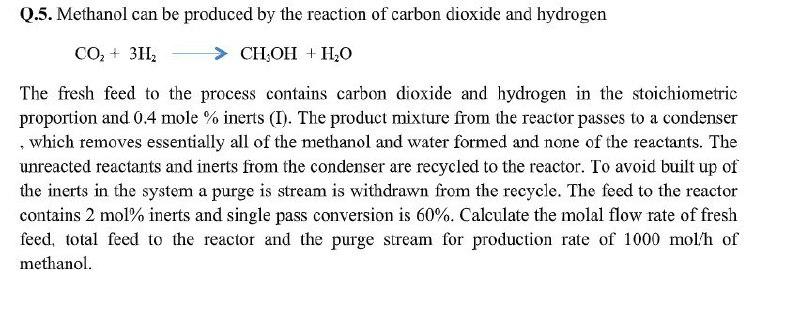
Q.5. Methanol can be produced by the reaction of carbon dioxide and hydrogen CO2 + 3H, CH-OH + H2O The fresh feed to the process contains carbon dioxide and hydrogen in the stoichiometric proportion and 0.4 mole % inerts (I). The product mixture from the reactor passes to a condenser , which removes essentially all of the methanol and water formed and none of the reactants. The unreacted reactants and inerts from the condenser are recycled to the reactor. To avoid built up of the inerts in the system a purge is stream is withdrawn from the recycle. The feed to the reactor contains 2 mol% inerts and single pass conversion is 60%. Calculate the molal flow rate of fresh feed, total feed to the reactor and the purge stream for production rate of 1000 mol'h of methanol
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


