Question: I need the answer in Matlab 7_3 (9 pts) In an m-file hw7_3.m, solve the following system of nonlinear equations with MATLAB. From the three
 I need the answer in Matlab
I need the answer in Matlab
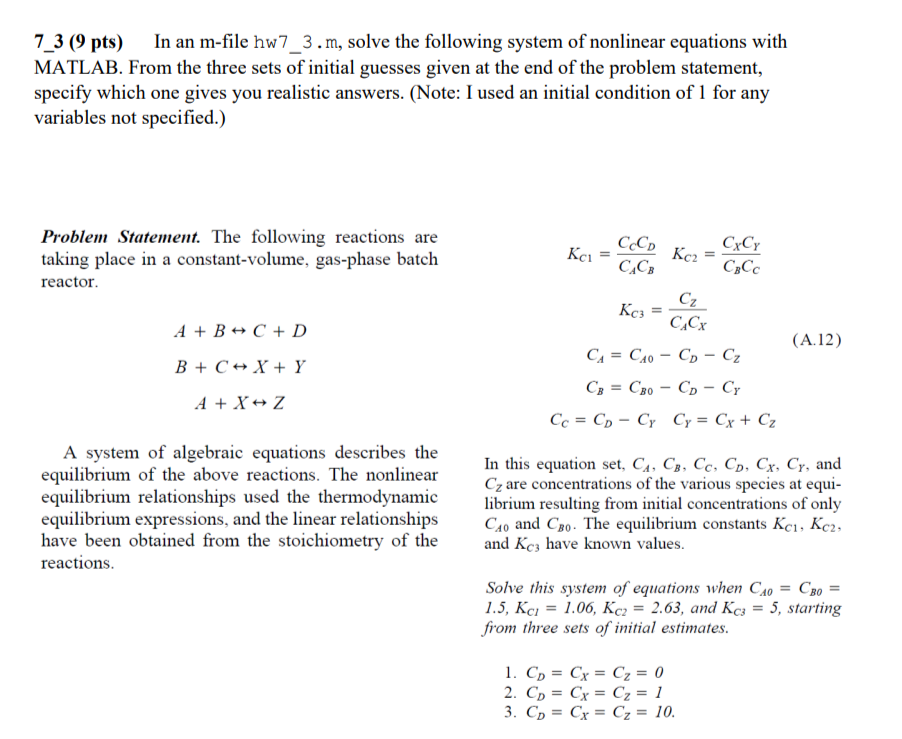
7_3 (9 pts) In an m-file hw7_3.m, solve the following system of nonlinear equations with MATLAB. From the three sets of initial guesses given at the end of the problem statement, specify which one gives you realistic answers. (Note: I used an initial condition of 1 for any variables not specified.) Problem Statement. The following reactions are taking place in a constant-volume, gas-phase batch reactor. = CCD CCB Kc2 CxCy Cz = A + B + C + D CACx (A.12) B + C + X + Y C = Cao - Cp - Ca CB = CBO - CD - Cy Cc = Cp - Cy Cy = Cx + Cz A + X Z A system of algebraic equations describes the equilibrium of the above reactions. The nonlinear equilibrium relationships used the thermodynamic equilibrium expressions, and the linear relationships have been obtained from the stoichiometry of the reactions. In this equation set, C4, C3, Cc, Cp, Cx, Cy, and Cy are concentrations of the various species at equi- librium resulting from initial concentrations of only Cao and Co. The equilibrium constants Kc, Kc2, and Kcz have known values. Solve this system of equations when C40 = CBo = 1.5, Kci = 1.06, Kc2 = 2.63, and Kc; = 5, starting from three sets of initial estimates. 1. Cp = Cy = Cz = 0 2. Co = Cx = C2 = 1 3. Cp = Cx = Cz = 10
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


