Question: I only had one? If so, can you please do one through six. Complete the balanced chemical reaction for the following weak base with a
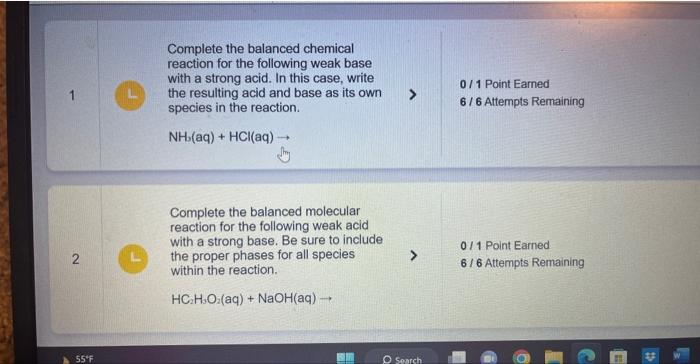
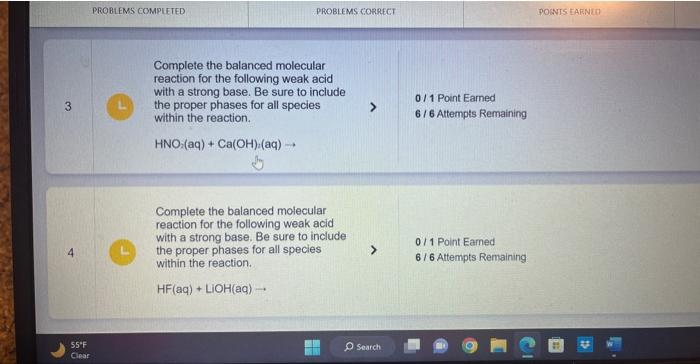
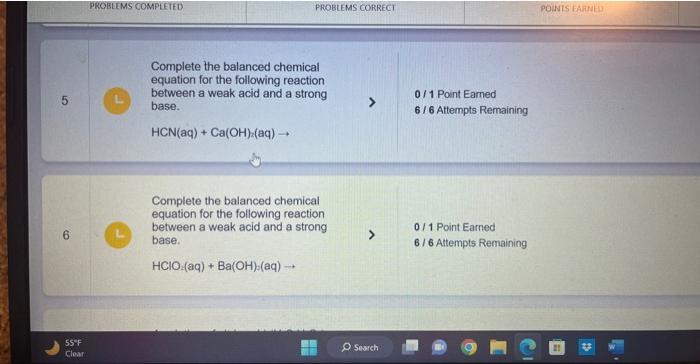
Complete the balanced chemical reaction for the following weak base with a strong acid. In this case, write the resulting acid and base as its own species in the reaction. NH(aq)+HCl(aq) Complete the balanced molecular reaction for the following weak acid with a strong base. Be sure to include the proper phases for all species within the reaction. HC2H3O2(aq)+NaOH(aq) Complete the balanced molec reaction for the following weal with a strong base. Be sure to the proper phases for all spec within the reaction. HF(aq)+LiOH(aq) Complete the balanced chemical equation for the following reaction between a weak acid and a strong base. HCN(aq)+Ca(OH)(aq) Complete the balanced chemical equation for the following reaction between a weak acid and a strong base. HClO2(aq)+Ba(OH)2(aq)
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


