Question: I only need help with B. I understand from other posts that the first-formed intermediate is more apt to revert to reactions, but I don't
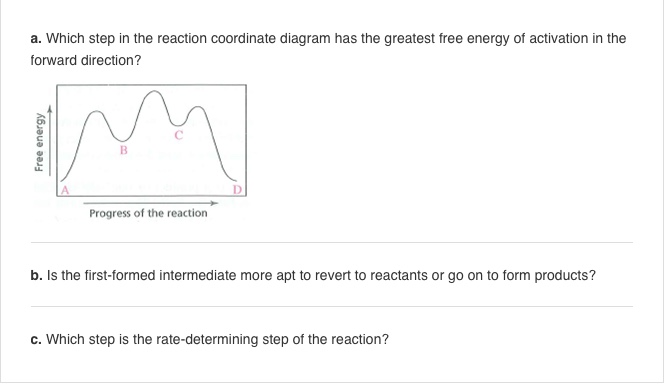
I only need help with B. I understand from other posts that the first-formed intermediate is more apt to revert to reactions, but I don't understand why.
a. Which step in the reaction coordinate diagram has the greatest free energy of activation in the forward direction? b. Is the first-formed intermediate more apt to revert to reactants or go on to form products? c. Which step is the rate-determining step of the reaction
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


