Question: I posted this question before but the answer was wrong. Would appreciate all steps, thank you so much! Q2) The component A was passed over
 I posted this question before but the answer was wrong. Would appreciate all steps, thank you so much!
I posted this question before but the answer was wrong. Would appreciate all steps, thank you so much!
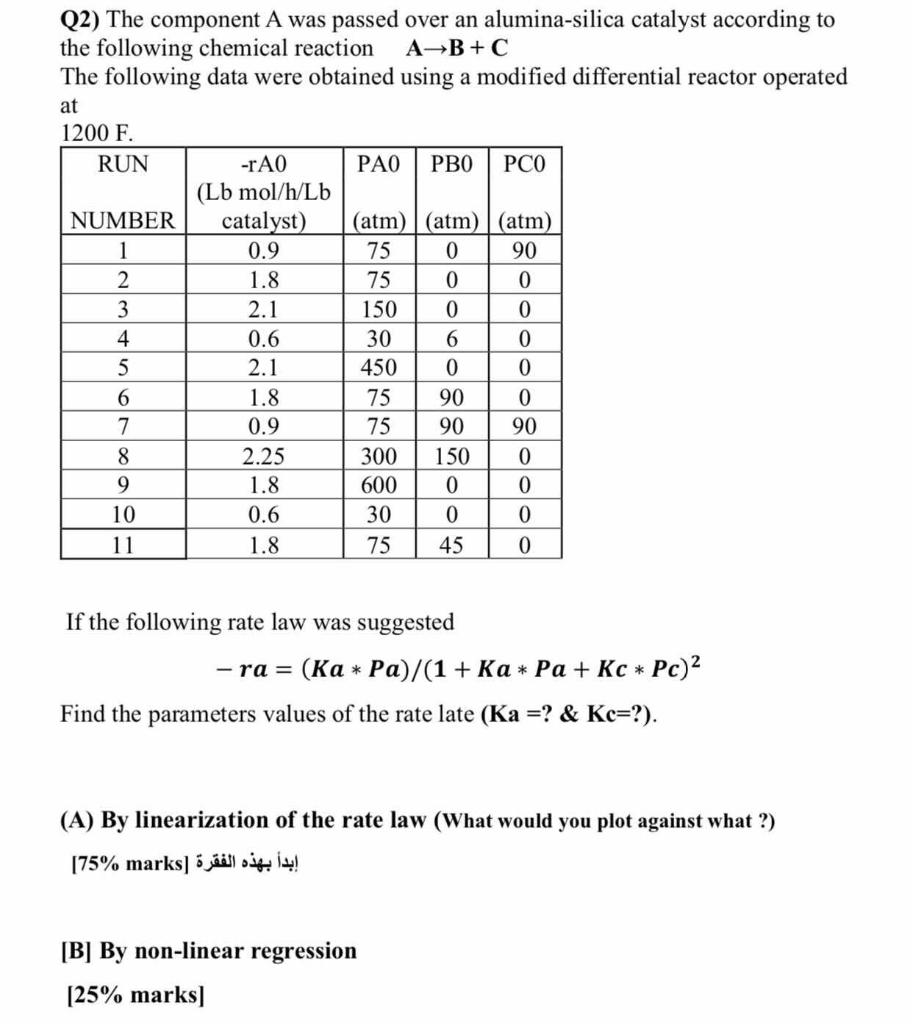
Q2) The component A was passed over an alumina-silica catalyst according to the following chemical reaction AB+C The following data were obtained using a modified differential reactor operated at 1200F. If the following rate law was suggested ra=(KaPa)/(1+KaPa+KcPc)2 Find the parameters values of the rate late (Ka= ? \& Kc= ?). (A) By linearization of the rate law (What would you plot against what ?) [75\% marks] [B] By non-linear regression [25\% marks]
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


