Question: I really just need a sample equation for each column. I only need rate constant, K A. Experimental Results Temperature 17.0C 10.5 Tahlo 1- Rato
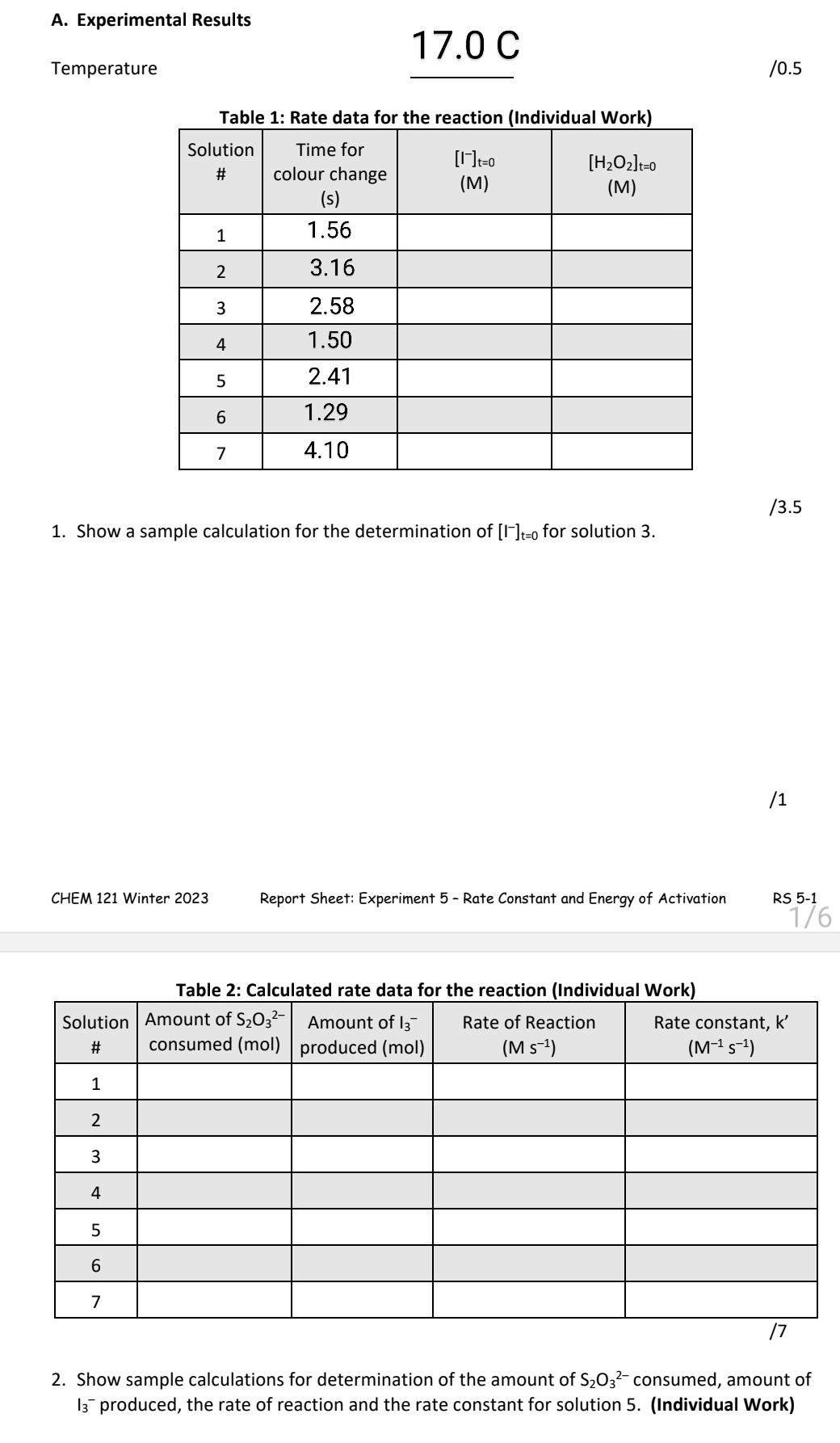
I really just need a sample equation for each column.
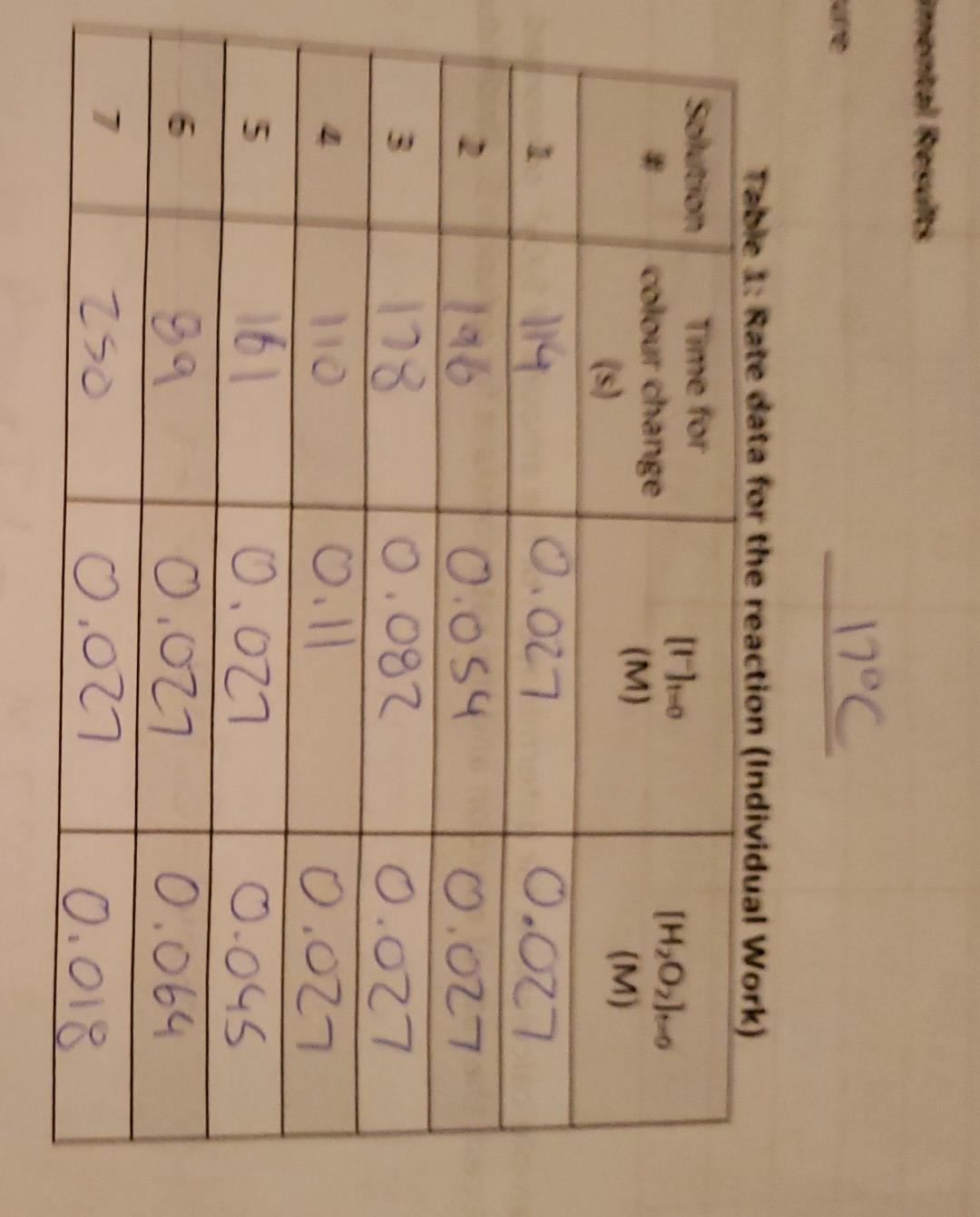
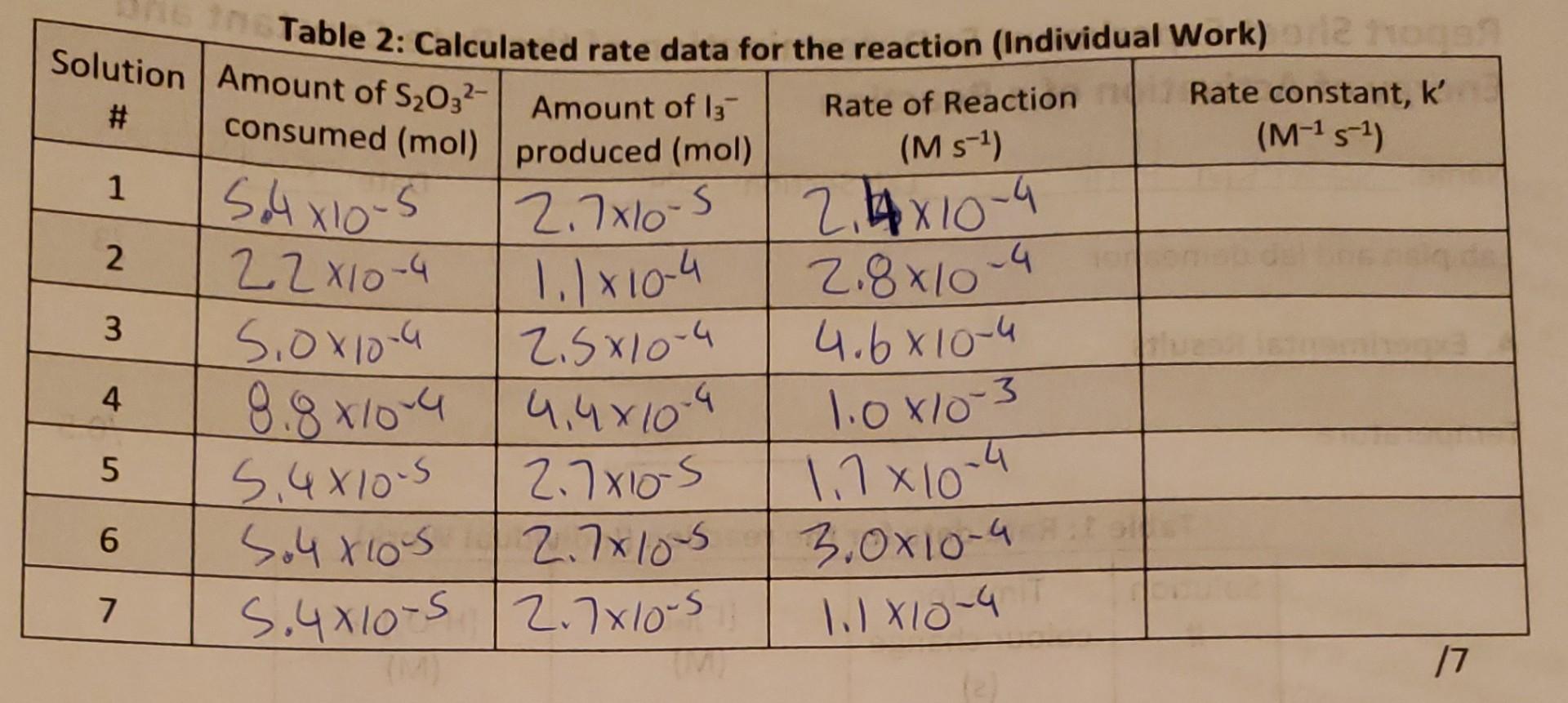
I only need rate constant, K
A. Experimental Results Temperature 17.0C 10.5 Tahlo 1- Rato data fnr tho roartinn (Individual Mork) 13.5 1. Show a sample calculation for the determination of [I]t=0 for solution 3 . /1 Report Sheet: Experiment 5 - Rate Constant and Energy of Activation RS 5-1 2. Show sample calculations for determination of the amount of S2O32 consumed, amount of I3produced, the rate of reaction and the rate constant for solution 5. (Individual Work) 1. Rate dafa for the reaction (Individual Work) Table 2: Calculated rate data for the reaction (Individual Work) \begin{tabular}{|c|l|l|l|l|} \hline Solution# & AmountofS2O3consumed(mol) & AmountofI3produced(mol) & RateofReaction(Ms1) & Rateconstant,k(M1s1) \\ \hline 1 & 5.4105 & 2.7105 & 2.4104 & \\ \hline 2 & 2.2104 & 1.1104 & 2.8104 & \\ \hline 3 & 5.0104 & 2.5104 & 4.6104 & \\ \hline 4 & 8.8104 & 4.4104 & 1.0103 & \\ \hline 5 & 5.4105 & 2.7105 & 1.7104 & \\ \hline 6 & 5.4105 & 2.7105 & 3.0104 & \\ \hline 7 & 5.4105 & 2.7105 & 1.1104 & \\ \hline \end{tabular} A. Experimental Results Temperature 17.0C 10.5 Tahlo 1- Rato data fnr tho roartinn (Individual Mork) 13.5 1. Show a sample calculation for the determination of [I]t=0 for solution 3 . /1 Report Sheet: Experiment 5 - Rate Constant and Energy of Activation RS 5-1 2. Show sample calculations for determination of the amount of S2O32 consumed, amount of I3produced, the rate of reaction and the rate constant for solution 5. (Individual Work) 1. Rate dafa for the reaction (Individual Work) Table 2: Calculated rate data for the reaction (Individual Work) \begin{tabular}{|c|l|l|l|l|} \hline Solution# & AmountofS2O3consumed(mol) & AmountofI3produced(mol) & RateofReaction(Ms1) & Rateconstant,k(M1s1) \\ \hline 1 & 5.4105 & 2.7105 & 2.4104 & \\ \hline 2 & 2.2104 & 1.1104 & 2.8104 & \\ \hline 3 & 5.0104 & 2.5104 & 4.6104 & \\ \hline 4 & 8.8104 & 4.4104 & 1.0103 & \\ \hline 5 & 5.4105 & 2.7105 & 1.7104 & \\ \hline 6 & 5.4105 & 2.7105 & 3.0104 & \\ \hline 7 & 5.4105 & 2.7105 & 1.1104 & \\ \hline \end{tabular}
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


