Question: i really need step by step on how to set this up with the numbers given for A: activation energy= 23.75 kj/mol delta E =
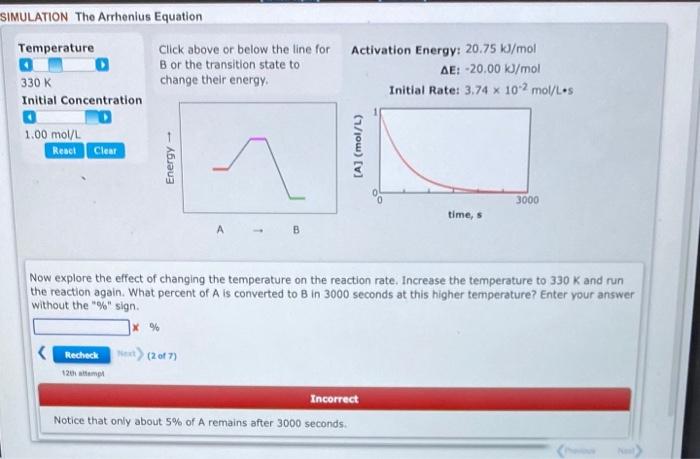
Click above or below the line for Activation Energy: 20.75kJ/mol B or the transition state to AE: 20.00k/mol change their energy. Initial Rate: 3.74102mol/Ls Now explore the effect of changing the temperature on the reaction rate. Increase the temperature to 330K and run the reaction again. What percent of A is converted to B in 3000 seconds at this higher temperature? Enter your answer without the "\%" sign. %
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


