Question: I tried getting help with this one before, but I've already tried half of the answers ive gotten on here and none have seemed to
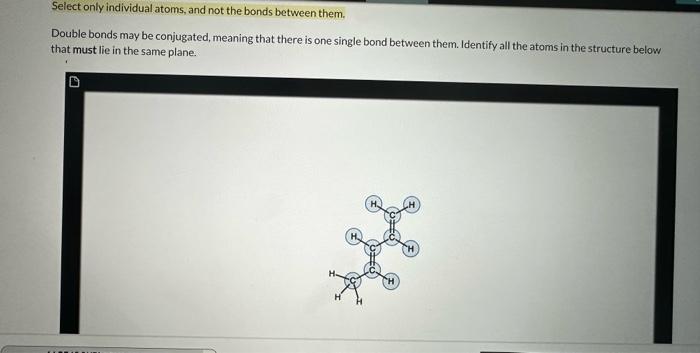

Select only individual atoms, and not the bonds between them. Double bonds may be conjugated, meaning that there is one single bond between them. Identify all the atoms in the structure below that must lie in the same plane. Although it is possible for this molecule to adopt a conformation in which both double bonds and all their attached atoms are in the same plane, it is not necessary for this to be the case. The single bond between the two double bonds can rotate freely, allowing the two trigonal planar systems to be out of plane with respect to each other without disrupting the structure
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


