Question: i want answer for all question (a) The reaction between sodium carbonate (Na2CO3) and silver nitrate (AgNO3) to form silver carbonate (Ag2CO3) and sodium nitrate
i want answer for all question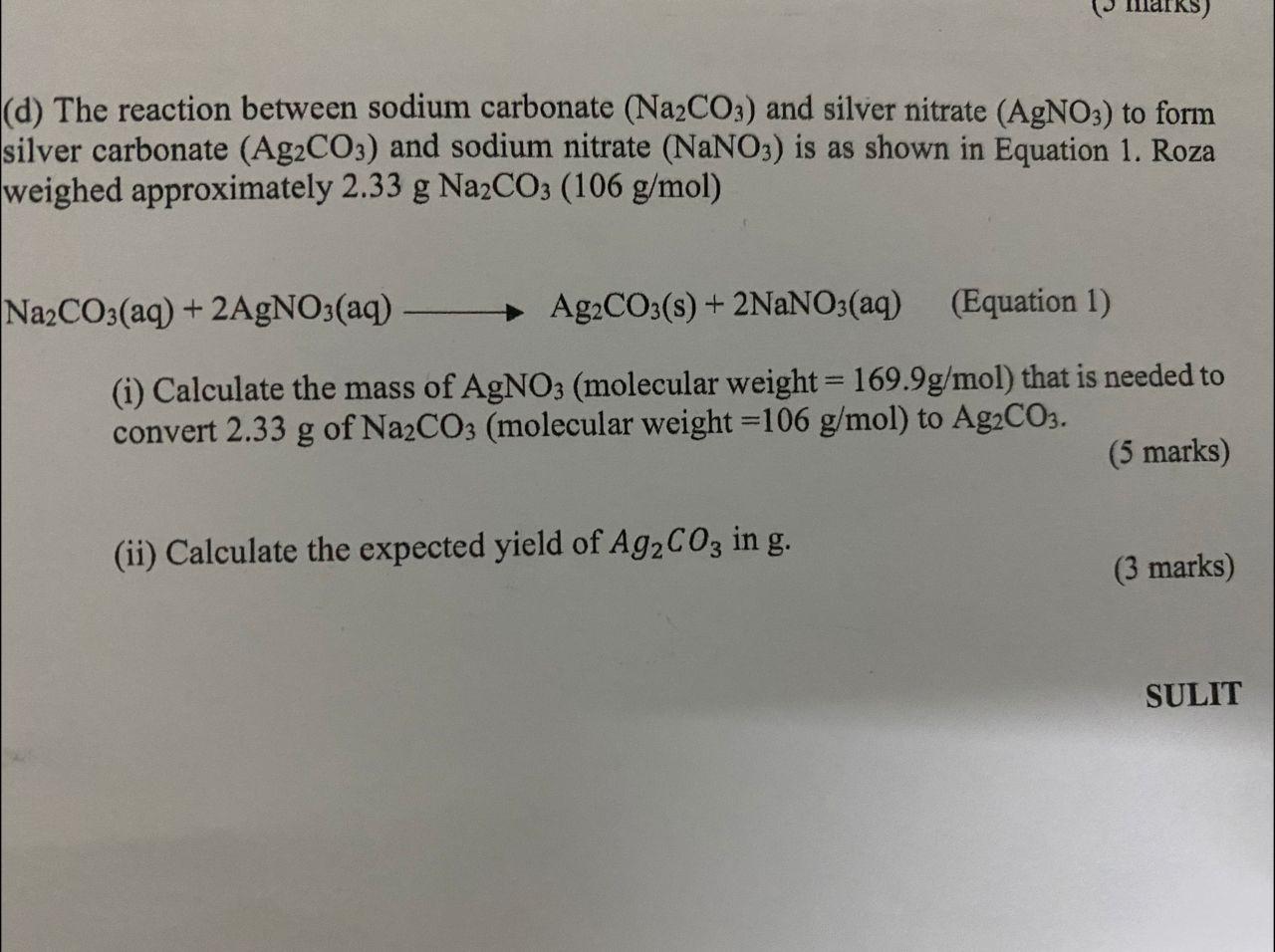
(a) The reaction between sodium carbonate (Na2CO3) and silver nitrate (AgNO3) to form silver carbonate (Ag2CO3) and sodium nitrate (NaNO3) is as shown in Equation 1. Roza weighed approximately 2.33 g Na2CO3 (106 g/mol) Na2CO3(aq) + 2AgNO3(aq) Ag2CO3(s) + 2NaNO3(aq) (Equation 1) (i) Calculate the mass of AgNO3 (molecular weight = 169.9g/mol) that is needed to convert 2.33 g of Na2CO3 (molecular weight =106 g/mol) to Ag2CO3. (5 marks) (ii) Calculate the expected yield of Ag2CO3 in g. (3 marks) SULIT
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


