Question: I want step by step explanation for each question please. I will give thumps up if I get the answer today before midnight. Thank you!
 I want step by step explanation for each question please. I will give thumps up if I get the answer today before midnight. Thank you!
I want step by step explanation for each question please. I will give thumps up if I get the answer today before midnight. Thank you!
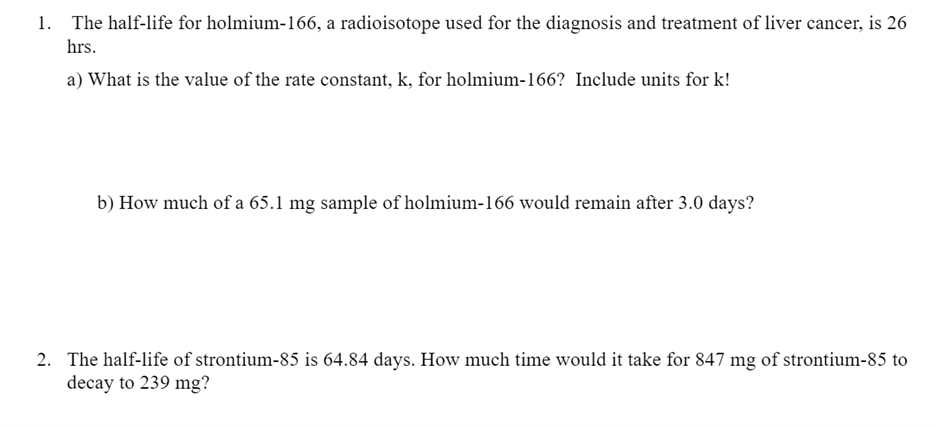
1. The half-life for holmium-166, a radioisotope used for the diagnosis and treatment of liver cancer, is 26 hrs. a) What is the value of the rate constant, k, for holmium-166? Include units for k ! b) How much of a 65.1mg sample of holmium-166 would remain after 3.0 days? 2. The half-life of strontium- 85 is 64.84 days. How much time would it take for 847mg of strontium- 85 to decay to 239mg
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


