Question: i want the answer for all question 1. A sample consisting of 2.00 mol Ar is expanded isothermally and reversibly at 0C from 22.4 dm'
i want the answer for all question
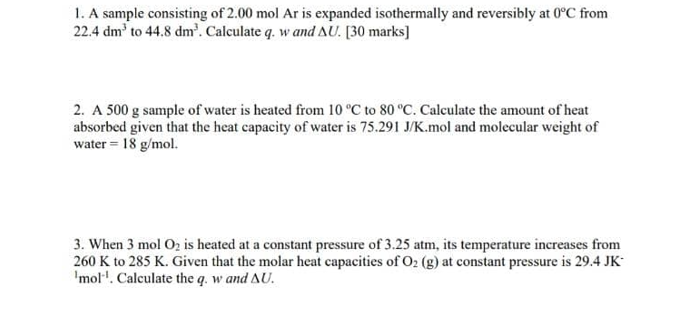
1. A sample consisting of 2.00 mol Ar is expanded isothermally and reversibly at 0C from 22.4 dm' to 44.8 dm'. Calculate q. w and AU. [30 marks] 2. A 500 g sample of water is heated from 10 C to 80 C. Calculate the amount of heat absorbed given that the heat capacity of water is 75.291 J/K.mol and molecular weight of water = 18 g/mol. 3. When 3 mol O2 is heated at a constant pressure of 3.25 atm, its temperature increases from 260 K to 285 K. Given that the molar heat capacities of O2 (g) at constant pressure is 29.4 JK 'mol. Calculate the q. w and AU
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


