Question: I WILL UPVOTE EXAMPLE: 4) Three unknown solutions were prepared by pipetting 25.00 mL of an unknown nickel solution into a 250.00 mL volumetric flask
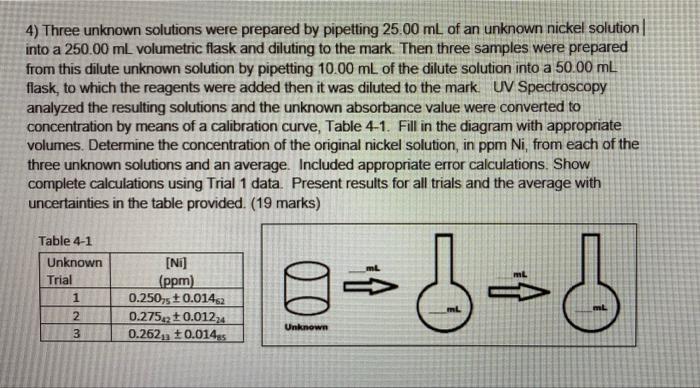
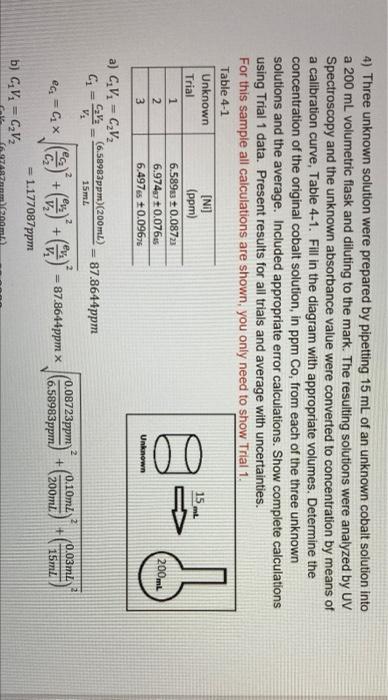
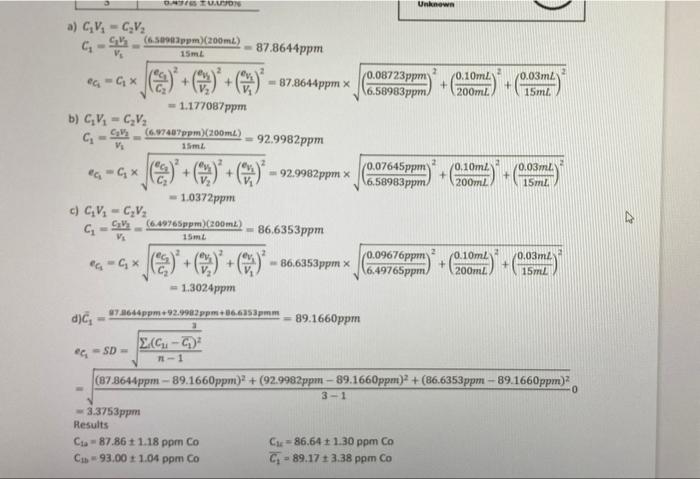
4) Three unknown solutions were prepared by pipetting 25.00 mL of an unknown nickel solution into a 250.00 mL volumetric flask and diluting to the mark. Then three samples were prepared from this dilute unknown solution by pipetting 10.00 mL of the dilute solution into a 50.00 mL flask, to which the reagents were added then it was diluted to the mark. UV Spectroscopy analyzed the resulting solutions and the unknown absorbance value were converted to concentration by means of a calibration curve, Table 4-1. Fill in the diagram with appropriate volumes. Determine the concentration of the original nickel solution, in ppm Ni, from each of the three unknown solutions and an average. Included appropriate error calculations. Show complete calculations using Trial 1 data. Present results for all trials and the average with uncertainties in the table provided. (19 marks) Table 4-1 Unknown Trial 1 2 [Ni] (ppm) 0.250,5 +0.01452 0.275,2 +0.01224 0.262, +0.014 gabob Unknown 3 4) Three unknown solution were prepared by pipetting 15 mL of an unknown cobalt solution into a 200 mL volumetric flask and diluting to the mark. The resulting solutions were analyzed by UV Spectroscopy and the unknown absorbance value were converted to concentration by means of a calibration curve, Table 4-1. Fill in the diagram with appropriate volumes. Determine the concentration of the original cobalt solution, in ppm Co, from each of the three unknown solutions and the average. Included appropriate error calculations. Show complete calculations using Trial 1 data. Present results for all trials and average with uncertainties. For this sample all calculations are shown, you only need to show Trial 1. Table 4-1 Unknown [NL] Trial (ppm) 1 6.58983 + 0.0872 2 6.974 +0.076 3 6.497s +0.0967 100 200mL Unknown a) C.V. = CV CV: (6.58983ppm)(200mL) V. 15 mL 87.8644ppm @=CX + () + = 87.8644ppm x 2 0.08723ppm) 0.10mZ + 16.58983ppm 200ml 0.03m2 15ml = 1.177087ppm b) GV = CV2 O TU. Unknown a) GV - CV (6.5ppm)(200 ml) Vi 15 m. G- 87.8644ppm ea-Gx + + **87.8644ppm x (0.08723ppm (6.58983 ppm. 10.10ml + 200m. + 0.03ml 15ml - 1.177087ppm b) CV - CV (6.17487ppm) (200ml) Vi 15m G- 92.9982 ppm =G***)+C) +C) - 92.9982pm x (0.07645ppm 16.58983ppm + 0.10ml 200ml 0.03m. 15ml -1.0372 ppm c) CV, -C,V: (6.49265ppm) (200 ml) VE 15 m. G-S 86,6353ppm ea-Gx + 2 + + 36,6353ppmx (0.09676ppm 6.49765ppm 0.10ml 200ml + 0.03m 15ml - 1.3024ppm d)c 9786ppm 92.9982 ppm36.6353pmm 89.1660ppm 2.6 - - SD -1 (87.8644ppm - 89.1660ppm) + (92.9982ppm - 89.1660ppm)2 + (86.6353ppm - 89.1660ppm) 0 3-1 3.3753ppm Results C-87.86 +1.18 pprn Co Cu = 86.64 + 1.30 ppm Co C1 - 93.00 +1.04 ppm Co Ci - 89.17 3.38 ppm Co
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


