Question: please check my work for the three sample calculations for the question in purple im not sure if i did these right at all, if




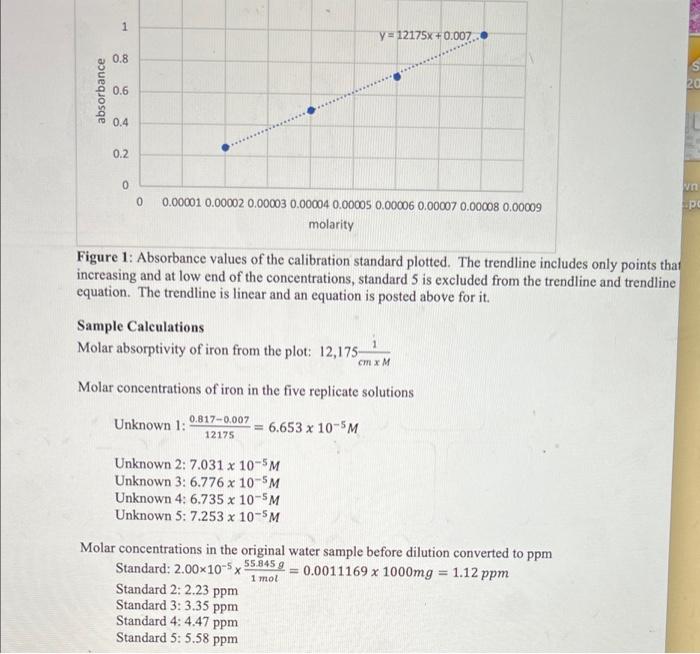
C. REAGENTS AND SOLUTIONS Stock solution of Fe?', 1.00x10? M Fe2+ in water Water sample containing unknown concentration of Fe2 Solution of 1,10-phenanthroline, 0.05% w/v in water Solution of ascorbic acid, 10 mM in water Sodium acetate to make a buffer, will be located by the top-loading balances. You will need to weigh an approximate amount. D. PROCEDURE 1. You are given a stock solution of 1.00x10-2 M Fe in water. Calculate what volume of this solution will be required to use dilution to prepare 100.0 mL of 1.00x10-MFe working stock solution. Verify this value with the instructor. Into the 50-ml beaker provided to you, obtain this volume (maybe twice as much, so you could more easily pipet it later). Clean and pre-rinse the correct volumetric pipet with this solution and perform the dilution into the 100.0-ml volumetric flask. This is your Fe* working stock solution, which will be used to prepare all calibration standards. 2. You will need to prepare -150 mL of buffer solution that is 4% w/v sodium acetate in water. Calculate the weight of sodium acetate needed and weigh this approximate amount on the top-loading balance. Transfer into a 250-ml beaker and, while stirring with a glass rod, gradually add Dl water to the 150-ml mark. Stir until dissolved. Cover with a watch glass until ready to use. 3. Obtain 60-70 mL of the 0.05% of the 1,10-phenanthroline solution in a 100-ml beaker. 4. Obtain 60-70 mL of the 10 mM ascorbic acid solution in a 100-ml beaker. 5. Calibration standards will be prepared in the 50-ml volumetric flasks provided. Rinse them all with Dl water, label 1-5. Reagents will be added, and the mixtures will be diluted to 50.00 ml. Calculate the volume of the working stock solution needed to prepare 50.00 mL of the following dilute solutions: 2.00x10-5 M 4.00x10 M, 6.00x105M, 8.00x10-5M, 1.0010 M Verify your calculations with the instructor. Decide what volumetric pipets you are going to use. Pre-rinse them with Dl water and then with the Fe?+ working stock solution; keep their tips resting over a clean paper towel to avoid contamination from the benchtop. Pipet the calculated amounts of the working stock into the flasks, but do not dilute to the mark until later. 6. Pre-rinse the last 50-ml volumetric flask for the blank and label "B" or "blank" or "O". Don't add iron solution. You will add all other reagents in the next step. (c) 5.0 mL of 0.05% phenanthroline solution - using a 10-ml graduated cylinder All these reagents will be present in excess; hence, the precision of graduated cylinders is enough. Keep the graduated cylinders and these reagent solutions for later. 8. Fill each flask to the mark with Dl water and invert several times: since there may not be enough stoppers or caps, cover the flask with a thumb (wear gloves). To minimize cross- contamination, proceed from the lowest concentration (blank) to the highest. 9. Allow all solutions to stand for 5 minutes to attain complete color development. In the meantime, rinse the six optical cuvets with Di water. Before you fill these cuvets (half-way) with the blank and standards, pre-rinse each cuvet with a sacrificial portion of the solution. The solutions should visibly show color progression with increasing Fe+ concentration. 10. Set the spectrophotometer to 510 nm and use the blank solution to zero it. Make sure you are in the Absorbance mode, as evidenced by letter "A" displayed. 11. Measure the absorbance of all the calibration standards at the same wavelength. The values should display a trend of increasing absorbance with increasing Fe* concentration. Spectrophotometric measurement results for the five standards prepared above: Absorbance Standard 1 (lowest concentration...) 0.253 Standard 2 (next concentration .... 0.499 Standard 3 (next...) 0.720 Standard 4 (next...) 0.991 Standard 5 (highest concentration) 12. Show these values to the instructor before discarding any solutions. When approved, keep the blank (flask and cuvet), but discard the standards (from the flasks and cuvets) into the waste beaker. Rinse these flasks and cuvets with Dl water. 13. Obtain 140-150 mL of the assigned unknown water sample into a clean 250-ml beaker. Unknown C was assigned 14. The five cleaned 50-ml volumetric flask will be used to prepare five replicates of the unknown. Clean and pre-rinse the 25-mL volumetric pipet with the unknown water sample and pipet 25.00 mL of it into each flask (do not dilute to the mark until later). 15. Add the other three reagents in the proportions used to prepare the standards. Dilute to the mark and invert the solutions as before. 16. Use the same spectrophotometer and re-zero it again with your saved blank. Perform the absorbance measurements of the unknowns at 510 nm. 1.018 CHEM 225 Spectrophotometric measurement results for the five replicates of the unknown: Absorbance Flask 1 0.817 Flask 2 0.863 Flask 3 0.832 Flask 4 0.827 Flask 5 0.890 Determine the molar absorptivity of the iron(II)-1,10-phenanthroline complex from the plot. Using the trendline equation, which shows how absorbance (Y) depends on the Fe2+ molar concentration (x), calculate the molar concentrations of Fe2+ in the five replicate solutions you prepared in the 50-ml flasks from the unknown water sample. Calculate the molar concentrations in the original water sample before the dilution (also five values). Convert them to ppm Fe (= mg Fe+ 1 y = 12175x+0.007 0.8 20 absorbance 0.6 0.4 0.2 VO 0 0 pe 0.00001 0.00002 0.00003 0.00004 0.00005 0.00006 0.00007 0.00008 0.00009 molarity Figure 1: Absorbance values of the calibration standard plotted. The trendline includes only points that increasing and at low end of the concentrations, standard 5 is excluded from the trendline and trendline equation. The trendline is linear and an equation is posted above for it. Sample Calculations Molar absorptivity of iron from the plot: 12,175 Molar concentrations of iron in the five replicate solutions Unknown 1: 0.817-0.007 12175 6.653 x 10-5M Unknown 2: 7.031 x 10-5M Unknown 3: 6.776 x 10-5M Unknown 4:6.735 x 10-5M Unknown 5: 7.253 x 10-5M Molar concentrations in the original water sample before dilution converted to ppm Standard: 2.00x10-5x 55.845 9 = 0.0011169 x 1000mg = 1.12 ppm Standard 2: 2.23 ppm Standard 3: 3.35 ppm Standard 4: 4.47 ppm Standard 5: 5.58 ppm 1 mol
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


