Question: Ia. Compound A is more reactive than compound B in an electrophilic aromatic substitution at ortho position. Using resonance structures and text, explain this order
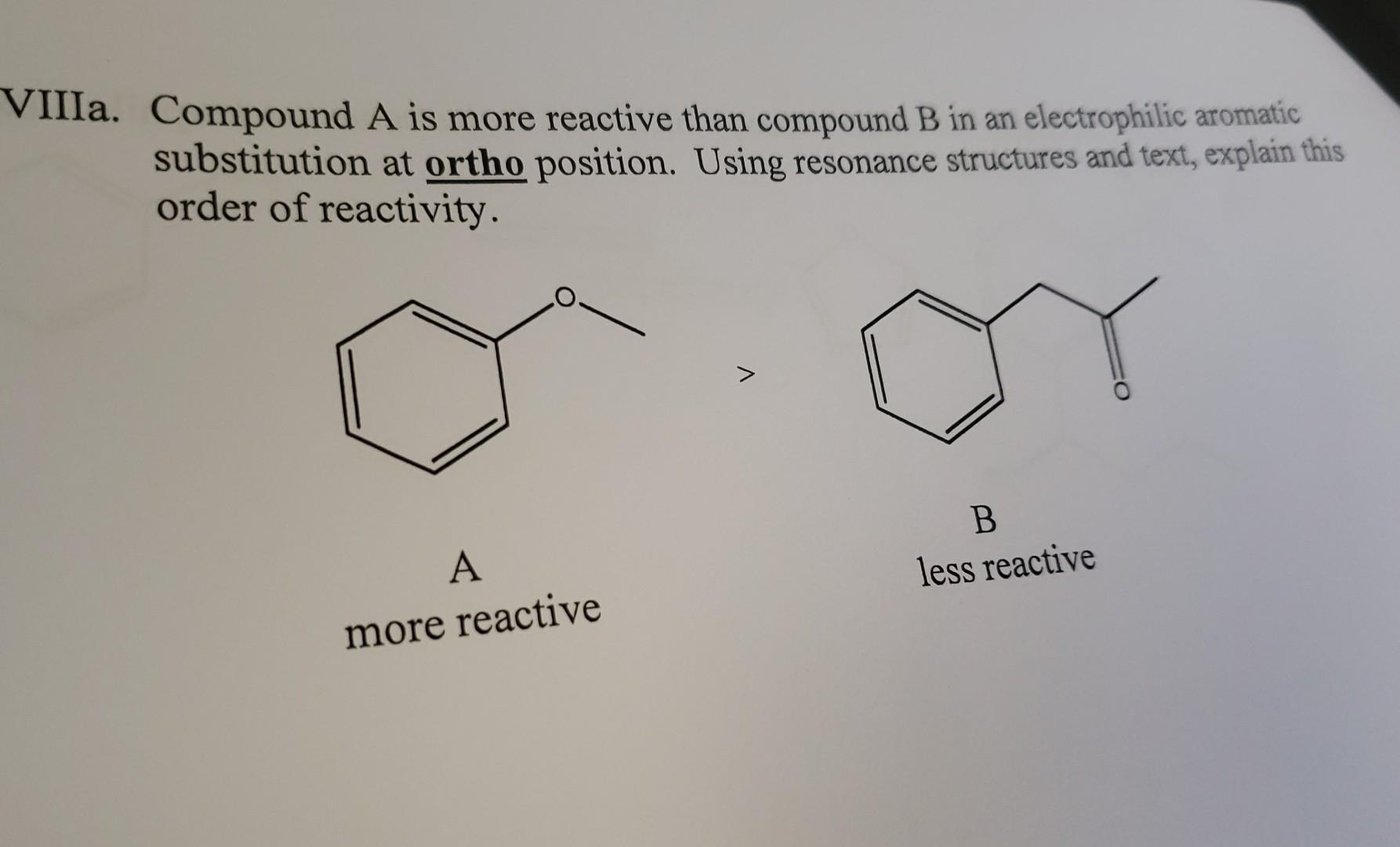
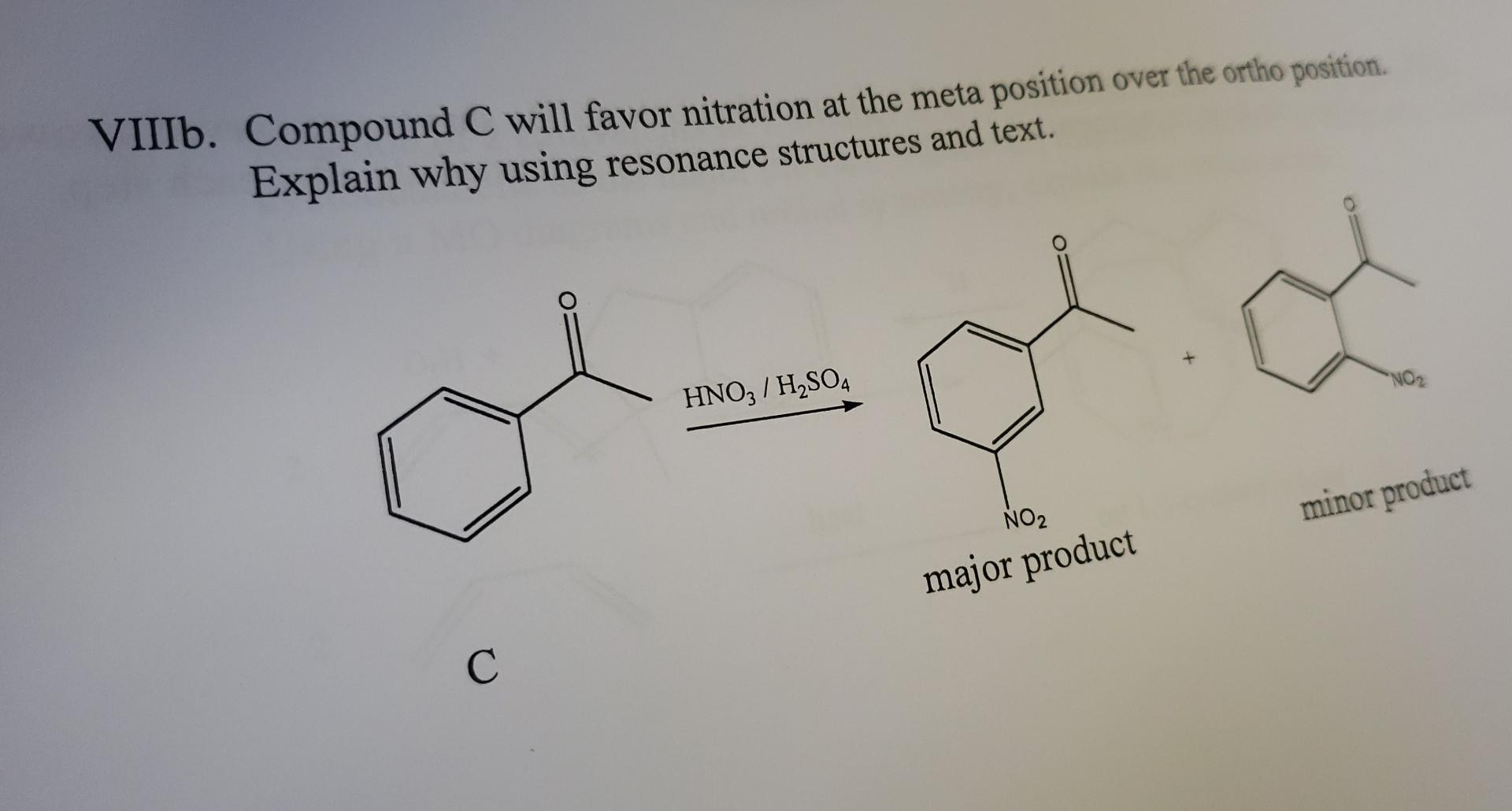
Ia. Compound A is more reactive than compound B in an electrophilic aromatic substitution at ortho position. Using resonance structures and text, explain this order of reactivity. VIIIb. Compound C will favor nitration at the meta position over the ortho position. Explain why using resonance structures and text. HNO3/H2SO4 minor product
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


