Question: ICE tables are used for calculating changes in concentration in an equilibrium system. I represents the initial concentration, C the change in concentration between the

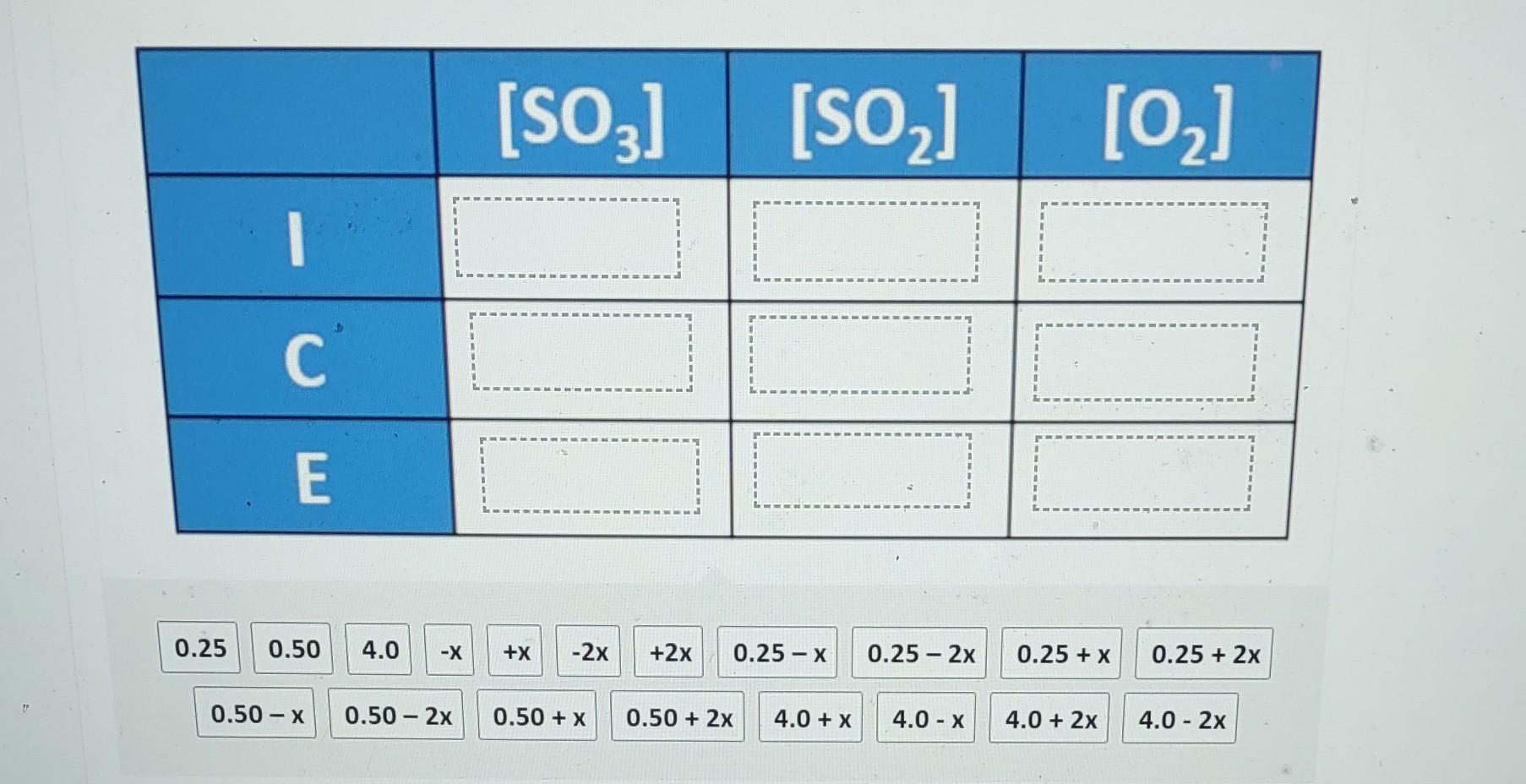
ICE tables are used for calculating changes in concentration in an equilibrium system. I represents the initial concentration, C the change in concentration between the initial value and the equilibrium value, and E the final concentration at equilibrium. The equilibrium constant for the following system is 0.0075 at 373K. 2SO3(g)2SO2(g)+O2(g) The initial concentrations of SO3,SO2, and O2 are 4.0M,0.25M, and 0.50M respectively. Complete the ICE table to show the changes and equilibrium concentrations using the values from the following list. \begin{tabular}{|c|c|c|c|} \hline & {[SO3]} & {[SO2]} & {[O2]} \\ \hline I & & & \\ \hline C & & & \\ \hline E & & & \\ \hline \end{tabular} 0.50x0.502x0.50+x0.50+2x4.0+x4.0x4.0+2x4.02x
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


