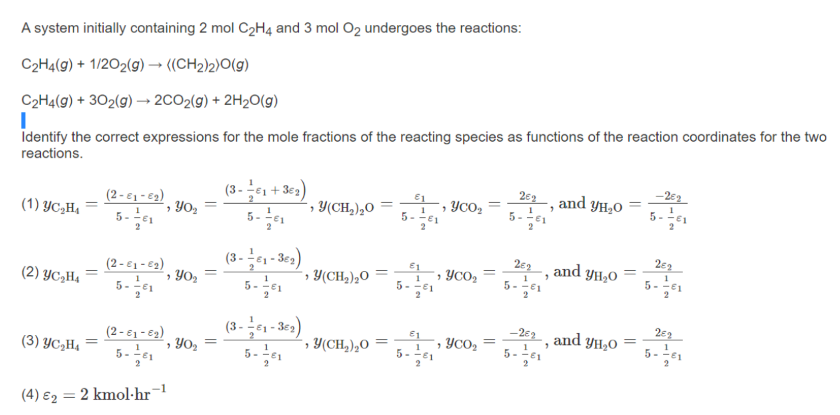
Question: Identify the correct expression 1, 2 ,3 ,or 4. A system initially containing 2molC2H4 and 3molO2 undergoes the reactions: C2H4(g)+1/2O2(g)(CH2)2O(g)C2H4(g)+3O2(g)2CO2(g)+2H2O(g) Identify the correct expressions for
Identify the correct expression 1, 2 ,3 ,or 4.
A system initially containing 2molC2H4 and 3molO2 undergoes the reactions: C2H4(g)+1/2O2(g)(CH2)2O(g)C2H4(g)+3O2(g)2CO2(g)+2H2O(g) Identify the correct expressions for the mole fractions of the reacting species as functions of the reaction coordinates for the two reactions. (1) yC2H4=5211(212),yO2=5211(3211+32),y(CH2)2O=52111,yCO2=521122, and yH2O=521122 (2) yC2H4=5211(212),yO2=5211(321132),y(CH2)2O=52111,yCO2=521122, and yH2O=521122 (3) yC2H4=5211(212),yO2=5211(321132),y(CH2)2O=52111,yCO2=521122, and yH2O=521122 (4) 2=2kmolhr1
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


