Question: Identify the correct statement pertaining to the structure shown below. The negative charge is localized on the oxygen. The negative charge on the oxygen would
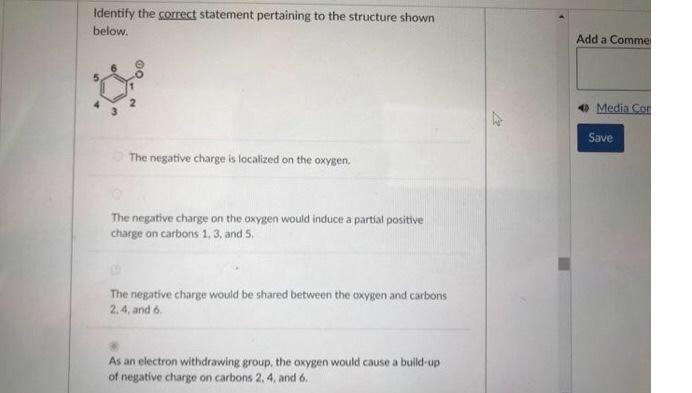
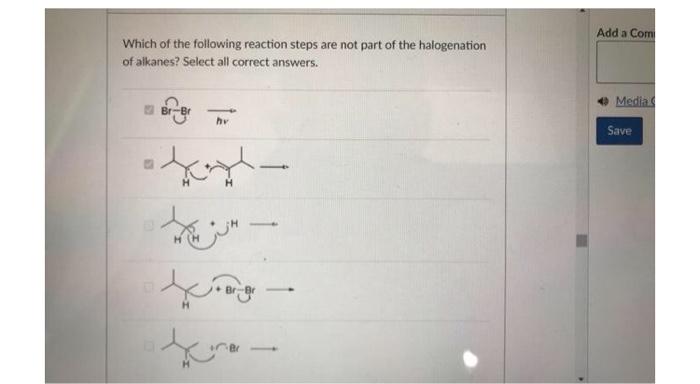
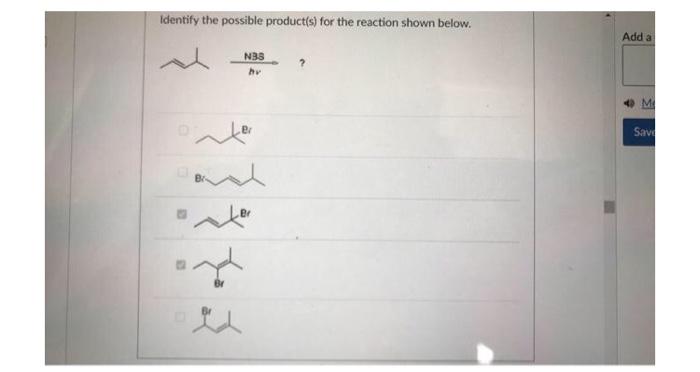
Identify the correct statement pertaining to the structure shown below. The negative charge is localized on the oxygen. The negative charge on the oxygen would induce a partial positive charge on carbons 1,3 , and 5 , The negative charge would be shared between the oxygen and carbons 2,4 , and 6 . As an electron withdrawing group, the oxygen would cause a build-up of negative charge on carbons 2, 4, and 6 . Which of the following reaction steps are not part of the halogenation of alkanes? Select all correct answers. Identify the possible product(s) for the reaction shown below. (i)
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


