Question: Identify the errors in these problems and EXPLAIN WHY they're wrong and the correct way to solve them. Thank you! 1. Suppose you mix 21.0g

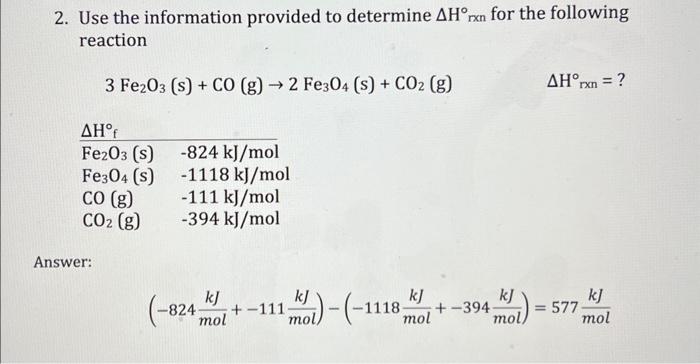
1. Suppose you mix 21.0g of water at 52.7C with 54.9g of water at 31.5 C in an insulated cup. What is the maximum temperature of the water after mixing? Answer: qsystem=qsurroundingsqhotH2O=qcoolH2OmhotH2OchotH2OThotH2O=mcoolH2OccoolH2OTcoolH2O(21.0g)(4.184gCJ)ThotH2O=(54.9g)(4.184gCJ)TcoolH2O(87.86CCJ)ThotH2O=(229.702oCJ)TcoolH2OThotH2O=(2.614)TcoolH2OTf52.7C=2.614Tf31.5C2.614Tf=21.2CTf=8.11C 2. Use the information provided to determine Hrxn for the following reaction 3Fe2O3(s)+CO(g)2Fe3O4(s)+CO2(g)Hrxn=? Answer: (824molkJ+111molkJ)(1118molkJ+394molkJ)=577molkJ
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


