Question: If an expert can please answer Part A and B questions by using calculations and showing your work. Also you must pay attention to the
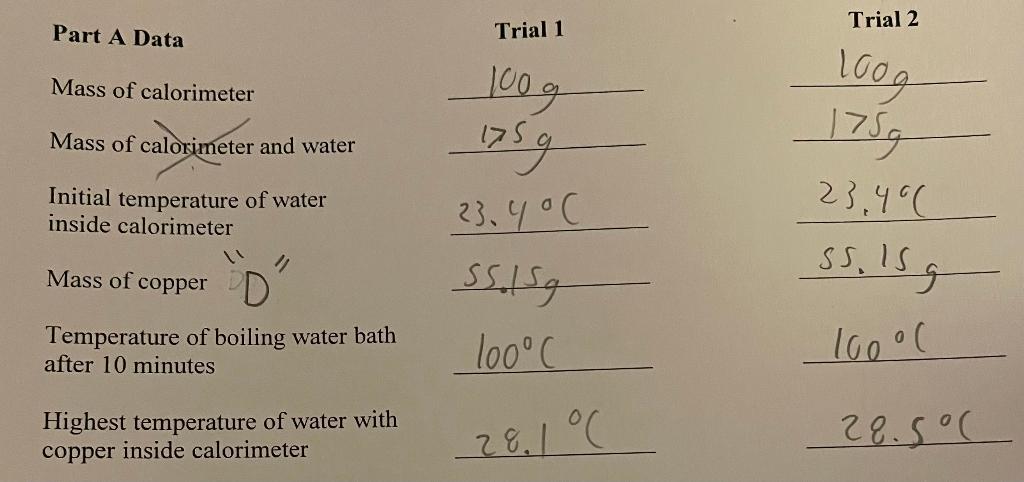

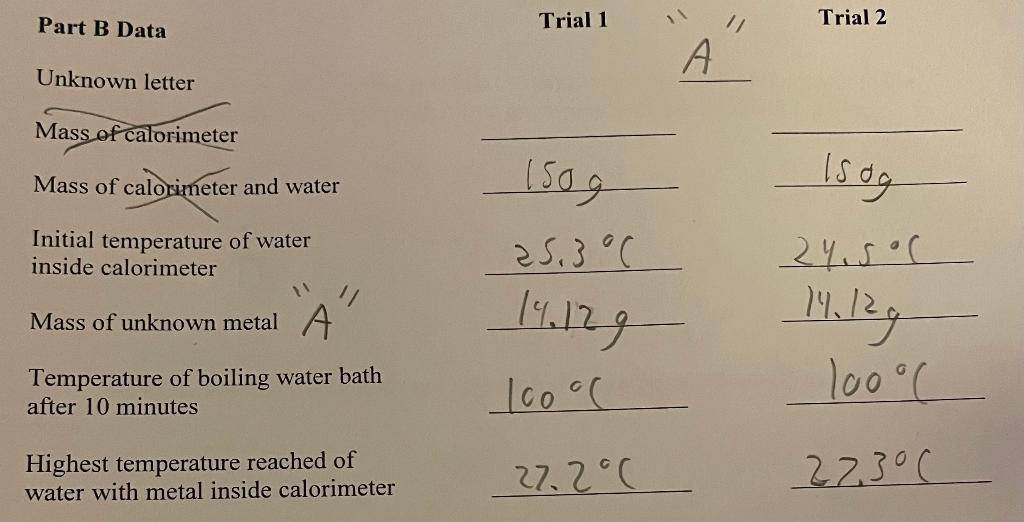
 If an expert can please answer Part A and B questions by using calculations and showing your work. Also you must pay attention to the significant figures. Also for question 5 from part B you must search up the table of select metals and their heat capacities. If an expert can please complete this as soon as possible I would really appreciate it please and thank you. This is for Chem 65.
If an expert can please answer Part A and B questions by using calculations and showing your work. Also you must pay attention to the significant figures. Also for question 5 from part B you must search up the table of select metals and their heat capacities. If an expert can please complete this as soon as possible I would really appreciate it please and thank you. This is for Chem 65.
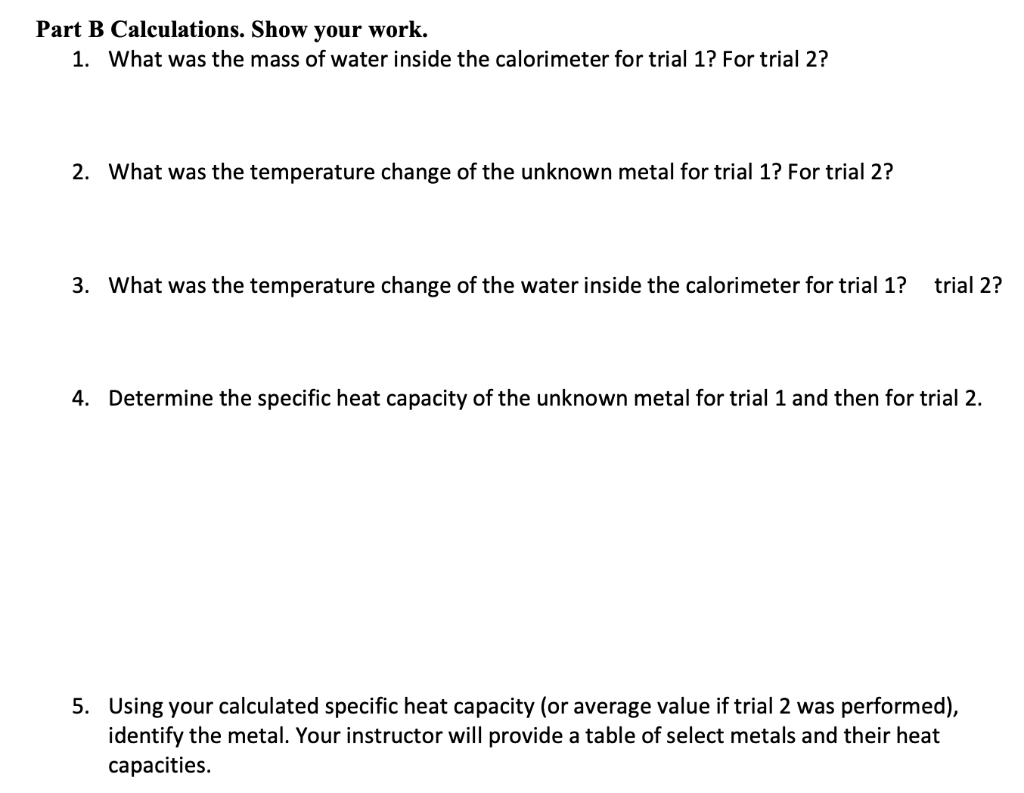
Part A Data \begin{tabular}{cc} Trial 1 & Trial 2 \\ 100g & 1009 \\ \hline 175g & 1759 \\ \hline \end{tabular} Initial temperature of water inside calorimeter Mass of copper D " 55.15923.40C 55.15923.46 Temperature of boiling water bath after 10 minutes 100C100C Highest temperature of water with copper inside calorimeter 28.1C 28.5C Part A Calculations. Show your work. 1. What was the mass of water inside the calorimeter for trial 1 ? For trial 2 ? 2. What was the temperature change of the copper for trial 1 ? For trial 2 ? 3. What was the temperature change of the water inside the calorimeter for trial 1 ? trial 2? 4. Determine the specific heat capacity of copper for trial 1 and then for trial 2 . 5. For trials 1 and 2, what was your average specific heat capacity of copper? Part B Data Trial 1 11/1 Trial 2 Unknown letter Mass of calorimeter Mass of calorimeter and water 4 Initial temperature of water inside calorimeter Mass of unknown metal A Temperature of boiling water bath after 10 minutes lsdg Highest temperature reached of water with metal inside calorimeter 14.12925.3C 14.12924.500 1001100C 27.2C27.3C art B Calculations. Show your work. 1. What was the mass of water inside the calorimeter for trial 1? For trial 2 ? 2. What was the temperature change of the unknown metal for trial 1 ? For trial 2 ? 3. What was the temperature change of the water inside the calorimeter for trial 1 ? trial 2 ? 4. Determine the specific heat capacity of the unknown metal for trial 1 and then for trial 2. 5. Using your calculated specific heat capacity (or average value if trial 2 was performed), identify the metal. Your instructor will provide a table of select metals and their heat capacities. 1. Which metal would have the greatest increase in temperature of the water in the calorimeter: the one with a higher or a lower specific heat capacity? Explain. 2. Relative to metals, how does the specific heat capacity of water compare? 3. If equal masses of two metals are heated to a temperature of 100C, which would cause a more sever burn: the one with the higher or lower specific heat capacity? Explain
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


