Question: If anyone can help complete this lab Questions and Problems Q.1 Complete the following table: [H3O+] [OH^] pH Acidic, Basic, or Neutral? 1 x 10-6
If anyone can help complete this lab
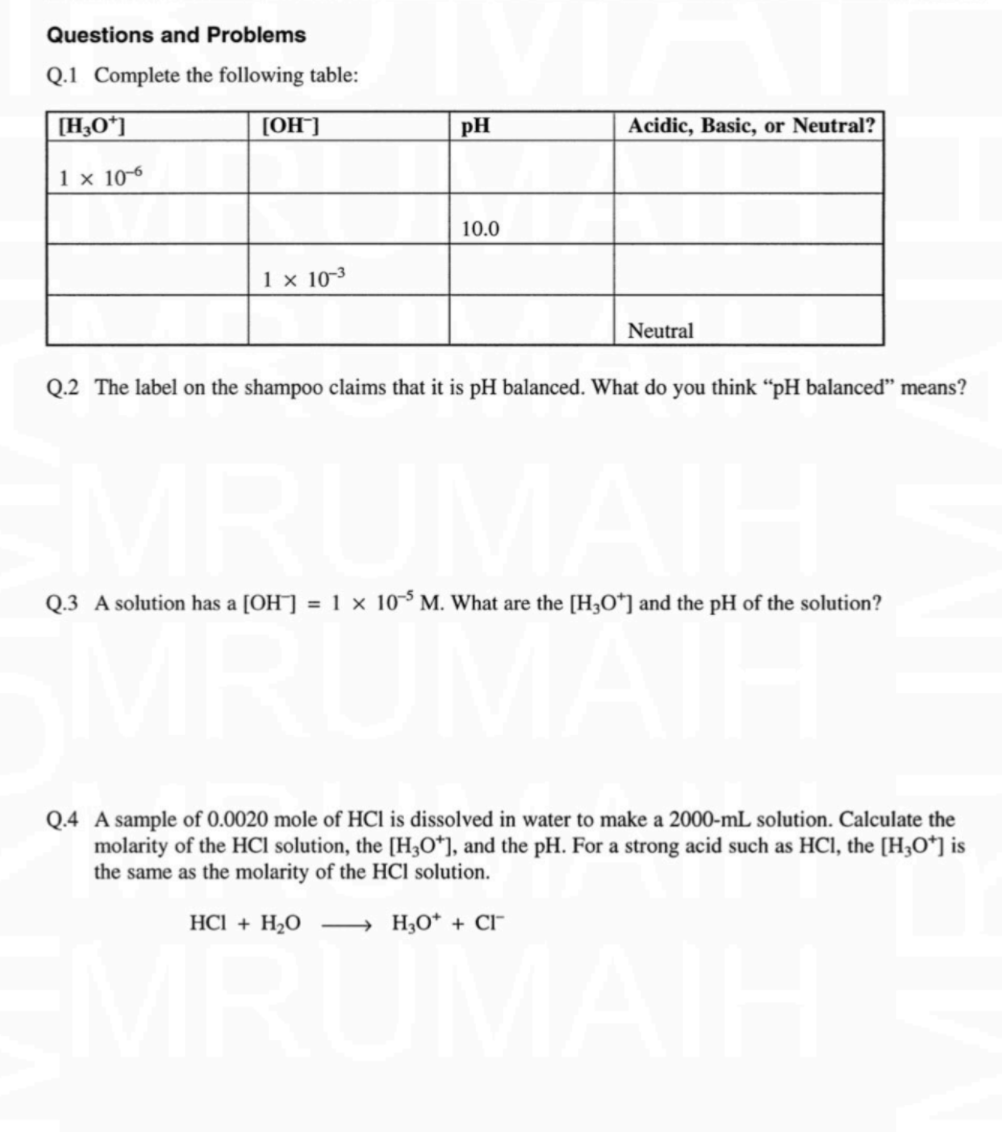
Questions and Problems Q.1 Complete the following table: [H3O+] [OH^] pH Acidic, Basic, or Neutral? 1 x 10-6 10.0 1 x 10-3 Neutral Q.2 The label on the shampoo claims that it is pH balanced. What do you think pH balanced means? Q.3 A solution has a [OH] = 1 x 10-5 M. What are the [H30*) and the pH of the solution? = Q.4 A sample of 0.0020 mole of HCl is dissolved in water to make a 2000-ml solution. Calculate the molarity of the HCl solution, the [H20*), and the pH. For a strong acid such as HCl, the [Hz0*) is the same as the molarity of the HCl solution. HCl + H2O H0* + CI MAS
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


