Question: if it could be clear steps that would be best, thanks 2) What is the change in entropy of 8.31 moles of ideal diatomic gas
if it could be clear steps that would be best, thanks
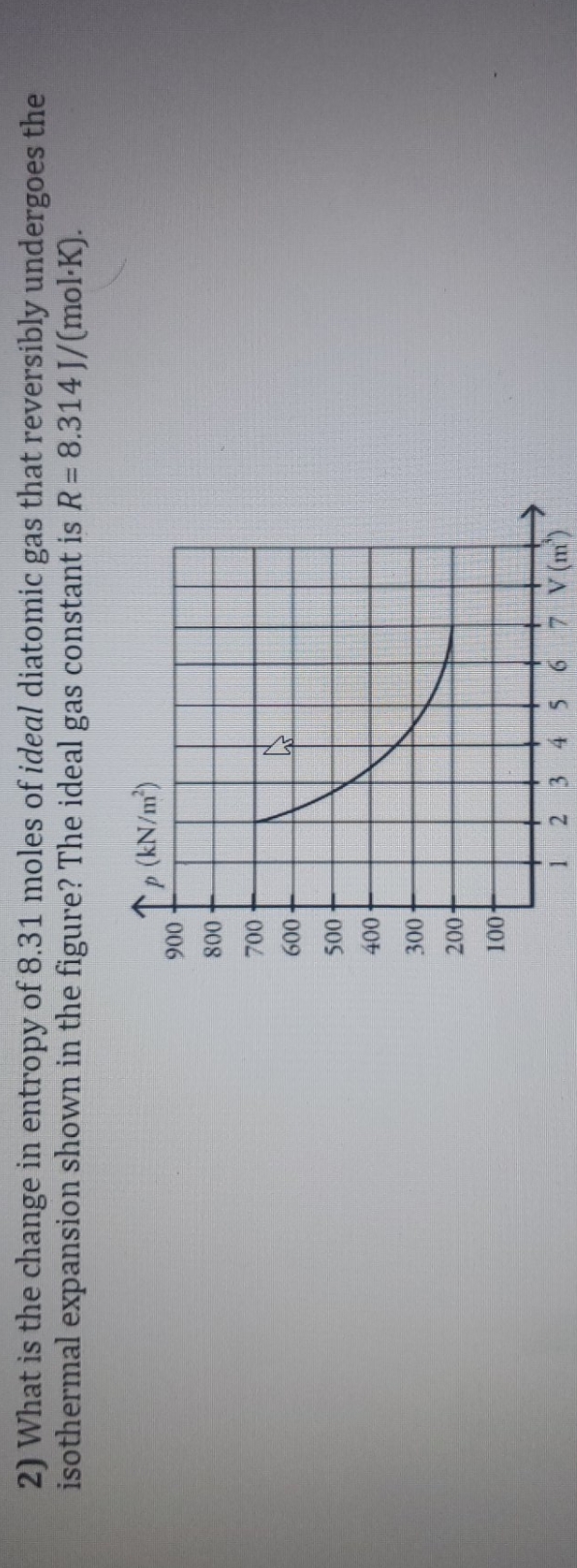
2) What is the change in entropy of 8.31 moles of ideal diatomic gas that reversible undergoes the isothermal expansion shown in the figure? The ideal gas constant is R = 8.314 ]/(mol. K). TP ( kN / m) 900 800 700 8 500 400 200 100 3 4 5 6 7 V (m
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


