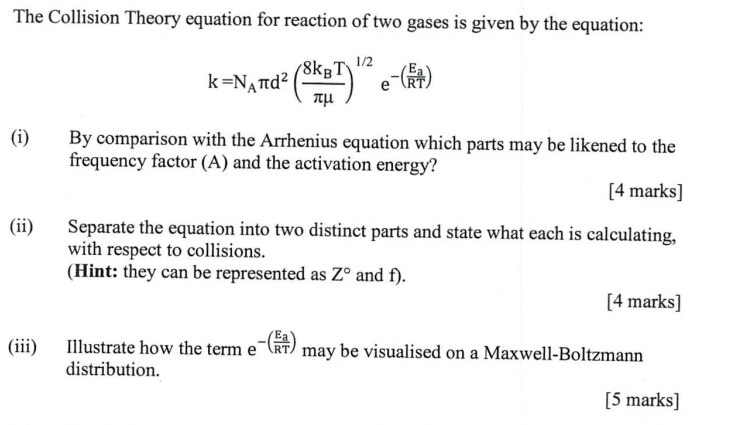
Question: if possible could u please explain also :) The Collision Theory equation for reaction of two gases is given by the equation: k=NAd2(8kBT)1/2e(RTEa) i) By
 if possible could u please explain also :)
if possible could u please explain also :)
The Collision Theory equation for reaction of two gases is given by the equation: k=NAd2(8kBT)1/2e(RTEa) i) By comparison with the Arrhenius equation which parts may be likened to the frequency factor (A) and the activation energy? [4 marks] ii) Separate the equation into two distinct parts and state what each is calculating, with respect to collisions. (Hint: they can be represented as Z and f ). [4 marks] iii) Illustrate how the term e e(RTEa) may be visualised on a Maxwell-Boltzmann distribution
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


