Question: If this is how the data is set up please just show what the code would look like! 5. (10 points) Download Hb.txt and read

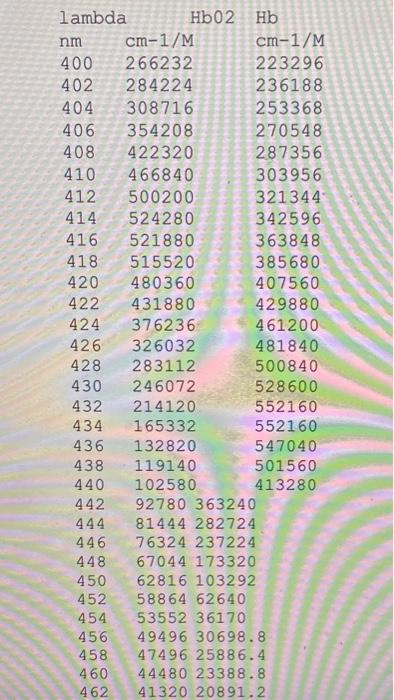
5. (10 points) Download Hb.txt and read in molar extinction coefficients [ cm1/( moles/liter)] of oxy-hemoglobin and deoxy hemoglobin at wavelengths from 400nm to 1000nm. Calculate absorption coefficients (cm1) for both oxy-hemoglobin (HbO) and deoxy hemoglobin (Hb) for all the wavelengths. Assume the gram molecular weight of hemoglobin ( HbO or Hb ) is 64,500[g/ mole]. Its concentration in blood is 150g/liter. Calculate and plot the absorption coefficients of deoxy-hemoglobin \& oxy-hemoglobin versus wavelength. Describe the major difference between these two curves. 5. (10 points) Download Hb.txt and read in molar extinction coefficients [ cm1/( moles/liter)] of oxy-hemoglobin and deoxy hemoglobin at wavelengths from 400nm to 1000nm. Calculate absorption coefficients (cm1) for both oxy-hemoglobin (HbO) and deoxy hemoglobin (Hb) for all the wavelengths. Assume the gram molecular weight of hemoglobin ( HbO or Hb ) is 64,500[g/ mole]. Its concentration in blood is 150g/liter. Calculate and plot the absorption coefficients of deoxy-hemoglobin \& oxy-hemoglobin versus wavelength. Describe the major difference between these two curves
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


