Question: IF YOU COULD ANSWER ALL 3 QUESTIONS, IT WOULD BE GREATLY APPRECIATED!:) THANK YOU Question 8 1 pts Using the table of standard enthalpies of
 IF YOU COULD ANSWER ALL 3 QUESTIONS, IT WOULD BE GREATLY APPRECIATED!:) THANK YOU
IF YOU COULD ANSWER ALL 3 QUESTIONS, IT WOULD BE GREATLY APPRECIATED!:) THANK YOU
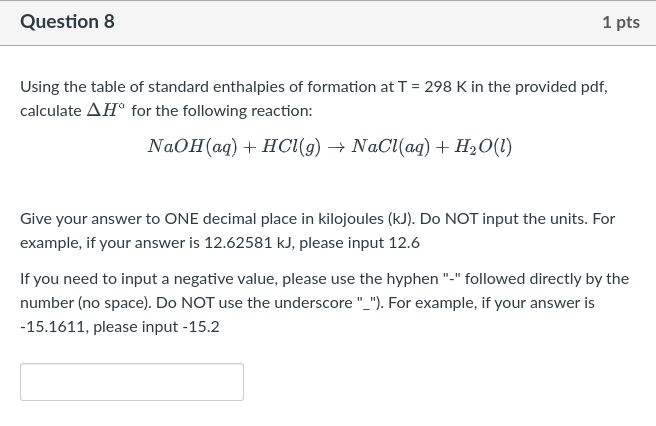
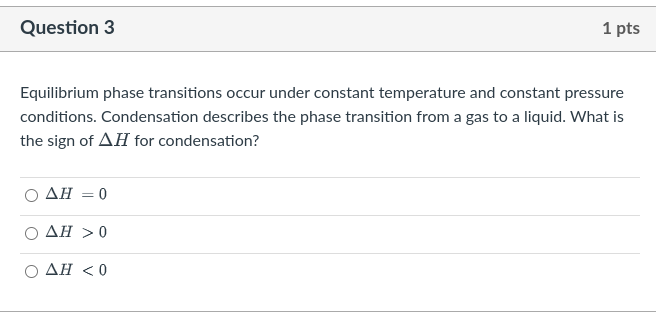
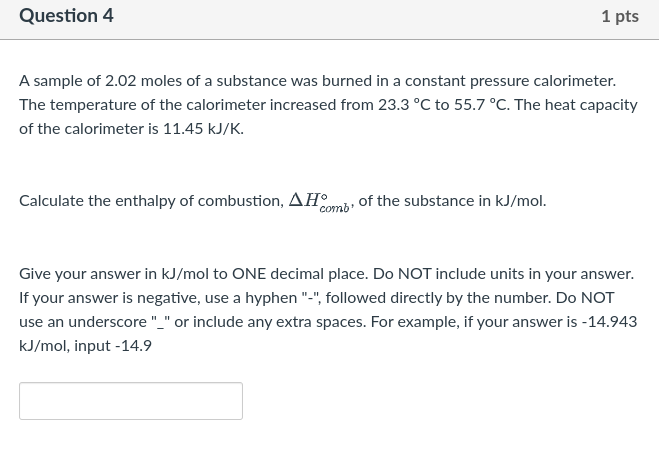
Question 8 1 pts Using the table of standard enthalpies of formation at T = 298 K in the provided pdf, calculate AH for the following reaction: NaOH(aq) + HCl(9) + NaCl(aq) + H2O(l) Give your answer to ONE decimal place in kilojoules (kJ). Do NOT input the units. For example, if your answer is 12.62581 kJ, please input 12.6 If you need to input a negative value, please use the hyphen "-" followed directly by the number (no space). Do NOT use the underscore "_"). For example, if your answer is -15.1611, please input-15.2 Question 3 1 pts Equilibrium phase transitions occur under constant temperature and constant pressure conditions. Condensation describes the phase transition from a gas to a liquid. What is the sign of AH for condensation? O AH = 0 AH > 0 AH
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


