Question: If you could show each step that would be greatly appreciated. Could you show the Excel work and VBA please? Thank you. = prap You
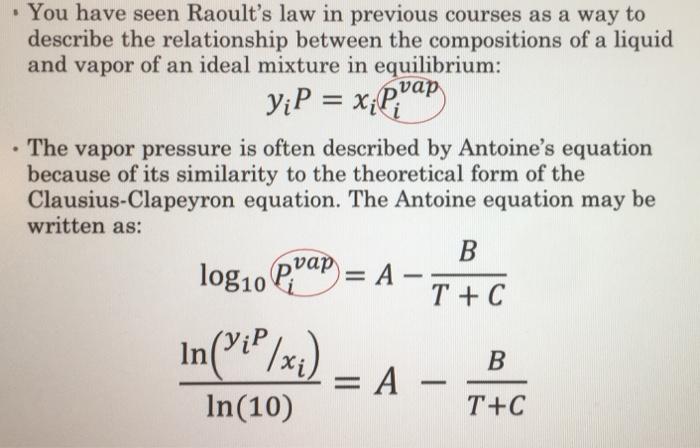


= prap You have seen Raoult's law in previous courses as a way to describe the relationship between the compositions of a liquid and vapor of an ideal mixture in equilibrium: yiP = xili" The vapor pressure is often described by Antoine's equation because of its similarity to the theoretical form of the Clausius-Clapeyron equation. The Antoine equation may be written as: B log10 Piap = A T +C ;P B = A - In(10) T+C In('_P/x+) Problem Statement Let's consider a situation where we introduce liquid 1,2-dichloroethane into an air-filled tank maintained at atmospheric pressure. The air above the liquid will become saturated with 1,2-dichloroethane vapor. The lower flammability limit (LFL) of 1,2-dichloroethane in air is 6.2 mol% and the upper flammability limit (UFL) of 1,2-dichloroethane in air is 16 mol%. (That is, if the vapor mole fraction is greater than 0.062 and less than 0.16, the mixture may flash in the presence of an ignition source.) Raoult's law may be considered to be appropriate in this case and you may assume that the liquid phase remains pure 1,2-dichloroethane. What temperature range should be avoided? Antoine's equation constants for 1,2-dichloroethane are: A = 7.02530 B = 1271.254 C = 222.927 These are valid for T between -30.8C to 99.4C when T is in C and P is in mm Hg. What you need to do Measure the barometric pressure and the ambient temperature Select between the graphing method, bisection method, Regula Falsi method, and the Newton-Raphson method to solve for the maximum temperature of this system using Raoult's law and the Antoine equation. Create VBA code to solve this. When you turn in your work, answer the following questions in the COMMENTS. What temperature will create a system at the LFL and what temperature will create a system at the UFL? Is this system likely to flash at ambient temperature in this lab? . = prap You have seen Raoult's law in previous courses as a way to describe the relationship between the compositions of a liquid and vapor of an ideal mixture in equilibrium: yiP = xili" The vapor pressure is often described by Antoine's equation because of its similarity to the theoretical form of the Clausius-Clapeyron equation. The Antoine equation may be written as: B log10 Piap = A T +C ;P B = A - In(10) T+C In('_P/x+) Problem Statement Let's consider a situation where we introduce liquid 1,2-dichloroethane into an air-filled tank maintained at atmospheric pressure. The air above the liquid will become saturated with 1,2-dichloroethane vapor. The lower flammability limit (LFL) of 1,2-dichloroethane in air is 6.2 mol% and the upper flammability limit (UFL) of 1,2-dichloroethane in air is 16 mol%. (That is, if the vapor mole fraction is greater than 0.062 and less than 0.16, the mixture may flash in the presence of an ignition source.) Raoult's law may be considered to be appropriate in this case and you may assume that the liquid phase remains pure 1,2-dichloroethane. What temperature range should be avoided? Antoine's equation constants for 1,2-dichloroethane are: A = 7.02530 B = 1271.254 C = 222.927 These are valid for T between -30.8C to 99.4C when T is in C and P is in mm Hg. What you need to do Measure the barometric pressure and the ambient temperature Select between the graphing method, bisection method, Regula Falsi method, and the Newton-Raphson method to solve for the maximum temperature of this system using Raoult's law and the Antoine equation. Create VBA code to solve this. When you turn in your work, answer the following questions in the COMMENTS. What temperature will create a system at the LFL and what temperature will create a system at the UFL? Is this system likely to flash at ambient temperature in this lab
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


